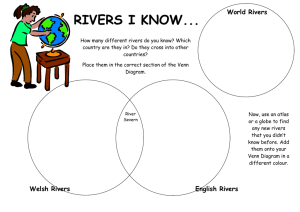
Ecological health assessment of large freswater rivers lakes and
advertisement

ECOLOGICAL HEALTH ASSESSMENT OF LARGE FRESHWATER RIVERS, LAKES AND RESERVOIRS: STUDY DESIGN OPTIONS, SAMPLING METHODS AND KEY ISSUES Prepared by the International WaterCentre Australia-China Environment Development Partnership River Health and Environmental Flow in China Project Code: P0018 20 February 2012 1 About this Document This document is one of a series of technical reports from the ACEDP River Health and Environmental Flow in China project. The project objectives are to document and trial, in China, international approaches to river health and environmental flows assessment. The trial involved three pilot river basins – the Yellow, Pearl and Liao River Basins. This review of methods applicable to large water bodies is not specific to the three pilot studies, although they all included river reaches that would be considered ʻlargeʼ. This review is of the global English language literature; while the findings and recommendations were not intended to be Chinaspecific, they are relevant to the design and implementation of river health assessment programs in China. Acknowledgements This report is the result of work undertaken as part of the River Health and Environmental Flow in China Project. The project forms part of the Australia-China Environment Development Partnership, a collaboration between the Australian and Chinese Governments, which is funded by the Australian Agency for International Development (AusAID). Recommended citation Leigh, C. and Gippel, C.J. 2011. Ecological Health Assessment of Large Freshwater Rivers, Lakes and Reservoirs: Study Design Options, Sampling Methods and Key Issues. Australia-China Environment Development Partnership, River Health and Environmental Flow in China. International WaterCentre, Brisbane, August. Disclaimer This publication may be of assistance to you, but the International WaterCentre and its employees and contractors do not guarantee that the publication is without flaw of any kind, or is wholly appropriate for your particular purposes and therefore disclaims all liability for any error, loss or other consequence which may arise from you relying on information in this publication. For further information on any of the information contained within this document contact: International WaterCentre Pty Ltd PO Box 10907, Adelaide St Brisbane, Qld, 4000 Tel: +61 7 31237766 Email: info@watercentre.org www.watercentre.org 2 Executive summary Assessment of condition and ecological health in freshwaters has been practiced across the globe for many years. Approaches to ecological assessment, however, have been most developed for small, wadeable streams rather than larger rivers. This is in part due to the sampling difficulties and other complexities posed by larger river systems but also in recognition of the many thousands of kilometers of tributary streams that are directly influenced by land use and in turn influence water quality downstream. In other large freshwater systems, such as lakes and reservoirs, monitoring has largely been focused on water quality, rather than a broad consideration of ecological health. There is, however, a growing need to identify and develop suitable methods for assessing the ecological health of large water bodies and their response to anthropogenic disturbance. This review focuses on study design options, sampling methods and key issues to consider in the assessment of ecological health in large water bodies. For assessment purposes, non-wadeable (‘large’) rivers are commonly defined by size criteria during the low [or average] flow periods when most sampling is conducted. China has thousands of reservoirs that offer aquatic habitat (albeit markedly different from that of the former flowing river), and many lowland rivers in China have associated large lowland lakes. This review includes such large water bodies (whether natural or artificial), defined here as lakes, reservoirs or wetlands that are non-wadeable during average hydrological conditions. Rivers that are under tidal influence, or which are estuarine, are not included in this review. Many river health assessment programs have adopted a ‘reference’ condition approach, using benchmarks derived from locations where human impact is low. Unfortunately, there are very few large water bodies that have escaped human impacts and that can be used as reference examples, and the ‘least disturbed’ sites in a highly disturbed system may appear similar to degraded sites. As a consequence, identifying the ‘best attainable’ condition may be the most appropriate target for many large water bodies. As with health assessments of small streams and other wadeable water bodies, the reference or target values that distinguish a ‘healthy’ system from an unhealthy one depend on the agreed objectives of the program and values to protect. Attributes of large water bodies that are relevant to ecological health can be measured using field sampling and/or remote sensing technologies. Field sampling of large water bodies, compared with smaller wadeable streams, often involves considerably more effort per site (due to the larger area that needs to be covered and logistical issues – e.g. need for boats or a working platform). The decision to include field sampling and choice of methodology will depend on project objectives, scope and standards, plus the physical heterogeneity of the water body in question. Where possible, pilot studies should be conducted to determine the sampling effort and costs of field measurements of physical, chemical and biological characteristics. The principles of monitoring hydrology for river health assessment are the same regardless of the size of the river, but in large rivers affected by weirs, it may also be important to measure water level variability (in addition to discharge variability). For lakes and reservoirs, the pattern of water level is of key hydrological concern. However, in some deeper systems it may also be necessary to monitor circulation and stratification, as affected by wind, inflows, 3 and thermal differences, as these have profound influence on water quality and the distribution of biota. The physical form of large rivers is difficult to measure objectively, with the visual estimation approach being particularly unreliable. The alternative is to measure surrogates (aspects of hydrology that are linked to channel stability) or to use remote sensing technologies. Some water quality parameters are important indicators of ecological health in their own right, and can in turn directly influence other (biotic) measures. Although regular field sampling is necessary for some aspects of water quality, much can be achieved through the use of continuously recording probes, and remote sensing technologies. Useful biotic indicator groups for large water bodies are algae, macroinvertebrates, fish, and littoral vegetation. Measures of aquatic primary production and respiration and other functional indicators (e.g. fish body condition) are also of relevance because they reflect important ecosystem processes. While algae, macroinvertebrates and fish require field sampling, remote sensing methodologies are well-established for measuring attributes of vegetation, and increasingly are being used to quantify levels of turbidity, primary production and other aspects of water quality and ecosystem function. Remote sensing is often more efficient than field sampling, but some indicators cannot be measured by remote sensing. In designing an ecological health assessment program for large water bodies it is necessary then to trade off the expediency of remote sensing against the detail that is possible with fieldbased sampling. In the context of large rivers, indicators of ecological health need to reflect relationships or responses to human disturbance as distinct from effects of river size. In large rivers heavily impaired by human disturbances, inclusion of dominant taxa in biotic measures (e.g. in those based on macroinvertebrates) may mask ecological responses to stressors and/or management actions, especially the responses of the more sensitive taxa. This becomes an issue for macroinvertebrate-based assessments when the substrate of the water body consists mainly of soft sediments and/or the few taxa found within the substrate are dominant taxa. However, in the assessment of lakes and reservoirs, the inclusion of indicators based on dominant biota, which include many toxic cyanobacteria species, may be of particular importance and relevance in ecological health assessments as phytoplankton blooms are often a key indication of an anthropogenically-disturbed lake ecosystem, and also are strongly linked to threats to human health. The design of a comprehensive river health assessment program for large water bodies and selection of indicators will ultimately depend on the program’s agreed objectives and values, and also on the likely threats to ecological integrity. It is important that the chosen indicators are useful in informing whether important management objectives are met or environmental values are protected. They must also be responsive to the likely threatening processes, such as changes in water quality or physical habitat. Ideally, the program should aim to use a mix of indicators that include measures of physical habitat (including hydrology), water quality, biota and important ecosystem processes. The choice of methods will also depend on available funding, and the skills of the staff. These considerations could lead to adoption of a program that includes both field-based and remote sensing approaches, plus a research component that seeks to continually develop the most cost-effective and robust methods. 4 Table of contents EXECUTIVE SUMMARY ....................................................................................................... 3 1. INTRODUCTION .......................................................................................................... 7 1.1. THE NEED TO INCLUDE LARGE WATER BODIES IN RIVER HEALTH ASSESSMENT ............................... 7 1.2. DEFINITION OF ‘LARGE WATER BODY’ ................................................................................... 7 1.3. SCOPE AND OUTLINE OF THIS REVIEW ................................................................................... 8 2. GUIDING PRINCIPLES FOR AN ECOLOGICAL HEALTH ASSESSMENT PROGRAM ........... 10 2.1. SETTING CLEAR GOALS AND/OR OBJECTIVES ........................................................................ 10 2.2. SELECTING APPROPRIATE INDICATORS FOR THE ASSESSMENT .................................................. 10 2.3. CLASSIFYING WATER BODIES INTO DIFFERENT TYPES .............................................................. 10 2.4. TESTING THE RESPONSE INDICATORS .................................................................................. 11 2.5. SETTING APPROPRIATE BENCHMARKS AND THRESHOLD VALUES FOR THE INDICES. ...................... 11 2.6. REPORTING ON THE RESULTS ............................................................................................ 12 2.7. IMPLEMENTING THE MONITORING PROGRAM TO INFORM MANAGEMENT. ............................... 13 3. PHYSICAL INDICATORS OF PRESSURES ...................................................................... 15 3.1. HYDROLOGY .................................................................................................................. 15 Hydrology of large rivers .................................................................................................. 15 Hydrology of lakes, wetlands and reservoirs .................................................................... 15 Remote sensing of hydrology ............................................................................................ 16 3.2. PHYSICAL FORM ............................................................................................................. 16 Field‐based assessment of river physical form ................................................................. 16 Indirect monitoring of river channel stability .................................................................... 17 Physical form of lakes, wetlands and reservoirs ............................................................... 19 Remote sensing of physical form ...................................................................................... 20 4. WATER QUALITY INDICATORS ................................................................................... 21 4.1. FIELD SAMPLING METHODS .............................................................................................. 21 4.2. CONTINUOUS MONITORING ............................................................................................. 22 4.3. REMOTE SENSING ........................................................................................................... 22 5. BIOTIC INDICATORS .................................................................................................. 23 5.1. DIATOMS AND OTHER ALGAE ............................................................................................ 23 5.2. MACROINVERTEBRATES ................................................................................................... 26 5.3. FISH ............................................................................................................................. 27 5 5.4. VEGETATION ................................................................................................................. 28 Field sampling ................................................................................................................... 28 Remote sensing ................................................................................................................. 29 5.5. ECOSYSTEM PROCESSES ................................................................................................... 29 5.6. SAMPLING EFFORT AND STUDY DESIGN IN FIELD‐BASED BIOASSESSMENTS ................................. 29 Large rivers ....................................................................................................................... 29 Large lakes and reservoirs ................................................................................................ 31 6. SELECTION OF APPROPRIATE AND RESPONSIVE INDICATORS .................................... 32 6.1. ABIOTIC INDICATORS ....................................................................................................... 32 6.2. BIOTIC INDICATORS ......................................................................................................... 32 7. CONCLUSIONS .......................................................................................................... 34 8. REFERENCES .............................................................................................................. 36 6 1. Introduction 1.1. The need to include large water bodies in river health assessment Assessment of condition and ecological health in freshwaters has been practiced across the globe for many years. Approaches to assessment, however, have tended to focus on and been developed for wadeable streams rather than large water bodies (i.e. low-gradient, high-order rivers that are nonwadeable, and large lakes and reservoirs) (Johnson et al. 1995). This is perhaps understandable, given that the health of large rivers and lakes ultimately depends on the influence of the numerous small and middle-sized streams that feed into them, which are in turn strongly influenced by adjacent land use. However, in addition to the cumulative impacts of land use on water quality from upstream catchments and tributaries, there are additional direct threats to the ecological integrity of large rivers and lakes from cities and industry (e.g. levees and channelization, dredging, and point source pollution, city effluent, turbulence from shipping etc.). The effects of multiple stressors, including non-point source pollution, on ecological integrity tend to accumulate from upstream to downstream, and can combine in large systems such that determining the individual sources of these effects becomes difficult (Blocksom and Flotemersch 2005). In addition to these multiple stressors, there are also often additional environmental and social values to address in larger rivers and lakes. This includes the consideration of large, charismatic aquatic species of conservation importance (e.g. river dolphins and migratory species such as sturgeon) and other species of commercial and recreational significance. There are often also growing community expectations in cities that the river or lake is clean and safe for human contact and other amenity values. The different stressors affecting large water bodies and additional environmental values and community expectations ultimately require additional or different indicators of river health than those typically used in the assessment of small streams. Furthermore, the physical, chemical and biological characteristics of freshwater systems change with size (e.g. Vannote et al. 1980, Chétalet et al. 2006) and many sampling protocols based on wadeable systems are impractical in large water bodies (Flotemersch et al. 2006a). Thus, overcoming the sampling difficulties and other complexities posed by large water bodies is necessary if large water bodies are to be included in river health assessment programs. There is a need to identify and document suitable methods for assessing river health and ecological response to anthropogenic disturbance in large water bodies. This review focuses on study design options and sampling methods suitable for, along with key issues to consider in, the health assessment of large water bodies. 1.2. Definition of ‘large water body’ One of the first issues to consider in the context of ecological health assessment of large water bodies is the definition of ‘large’. There are numerous classifications in the literature, but for the purposes of river health assessment, they appear to centre on two factors: first, that the river, at some point, is ‘nonwadeable’ across its breadth or along its length (although this may depend on hydrological conditions); and second that it is greater than some (arbitrary) measure of size, such as drainage area (e.g. ≥ 2 3 1600 km ), river length (e.g. ≥ 100 km), discharge (e.g. ≥ 15 m /s) or order (e.g. ≥ 5) (Wilhelm et al. 2005). Wilhelm et al. (2005) recommended that for assessment purposes, non-wadeable (‘large’) rivers should be defined by one or more such size criteria and by “a boundary that, on average, defines a river reach that is non-wadeable during the low [or average] flows when most sampling occurs.” Kellerhals and Church (1989) classified ‘large rivers’ as ones unlikely to be affected locally by blockage due to fallen trees, in which individual sediment grains were insignificant as form elements, and in which the relative roughness (ratio of bed particle diameter to flow depth) was less than 0.3. The transition to 7 3 -1 large channels, on these criteria, falls somewhere near a bankfull discharge of 20 m s , and channel width of 20 m. Importantly, most of these criteria are somewhat arbitrary from an ecological standpoint, being more based on sampling methodologies – a fact evidenced by the strong distinction between ‘wadeable’ and ‘non-wadeable’ streams in numerous assessment programs. Simple hand or foot operated sampling methods, which were developed for studies of small streams, are not feasible in ‘non-wadeable’ and alternative methods using boats or pontoons are required. Rivers with sizeable catchments may be large (i.e. non-wadeable) in their freshwater reaches, while smaller rivers might become large in their estuarine reaches. Rivers that are under tidal influence, or which are estuarine, are not included in this review. This is because the complex hydrodynamic and salinity regimes of estuaries, plus the common presence of accumulated multiple stressors, requires application of a different approach to health assessment compared with freshwater rivers. The differences are large enough that the topic of health assessment of estuaries warrants separate documentation. Large lentic water bodies (lakes, reservoirs and other wetlands) can be present on river systems, even on wadeable rivers. China has thousands of reservoirs that offer aquatic habitat (even if very different from that of the former flowing river), and lowland rivers in China typically have associated large lowland lakes (Liu and Wang 2010). This review includes such large water bodies (whether natural or artificial). Here we apply a modification of the definition Wilhelm et al. (2005) suggested for rivers, as a boundary that, on average, defines a lake, reservoir or wetland that is non-wadeable during average hydrological conditions. A large wetland would be classified as ‘permanent’. 1.3. Scope and outline of this review Much of the published literature on non-wadeable river assessment has been based on North American rivers (e.g. Lazorchak et al. 2000) and has focused on the use of multi-metric biotic indices to assess river health (e.g. Flotemersch et al. 2006b, Weigel and Dimick 2011). Despite this Nth American focus, the key issues identified by these studies are more broadly applicable and can be summarized as: • the lack of reference conditions in many large water bodies; • the appropriate scale required to define human disturbances and the selection of appropriate measures of human disturbance; • the most suitable and effective methods for surveying abiotic parameters and characterizing different groups of biota (e.g. algae, macroinvertebrates, fish) to detect ecological responses to human disturbance, including considerations of site comparability, habitat selection, and sampling effort; and • the most appropriate and responsive indicators (in terms of response to human disturbances and management). In this review, general principles on river health assessment are first summarized to provide the necessary context. Then, each of the above topics is reviewed and discussed below, followed by some general conclusions and recommendations. This review assumes that the reader has prior knowledge and general understanding of health assessment philosophy and practices. These have been covered thoroughly in literature such as Boulton (1999) and other papers in this issue of Freshwater Biology (volume 41), Flotemersch et al. (2006b), Bunn et al. (2010), and in the ACEDP river health assessment framework (Gippel 2010). In addition, it is important to note that this review is not intended to be prescriptive in terms of which methods and indicators should be used to develop a health assessment program for large water bodies. Indeed, many of the above issues, which are elaborated in this review, can be resolved through consideration of the objectives and scale of interest of the river health assessment program in question (see section 2, guiding principles, below,). Therefore, it is important that these criteria (objectives and scale of interest) are established prior to site selection and sample collection, along with other important steps and elements recommended when formulating an assessment program, such as catchment-scale 8 river classification, conceptual model development, identification of ecological assets and the major threats to river health, and data quality assurance and control (e.g. Flotemersch et al. 2006b; Bunn et al. 2010). Finally, if information on causality of poor ecological condition is required, which may be particularly relevant in terms of management or restoration, then methods to help distinguish among effects of multiple stressors and to detect unacceptable levels of change may need consideration (Downes et al. 2002; Downes 2010). 9 2. Guiding principles for an ecological health assessment program 2.1. Setting clear goals and/or objectives It is important to be clear as to why you are monitoring and what you wish to achieve. Is it to protect important environmental assets and values (e.g. conserve wetlands)? Is it to maintain ecosystem goods and services (e.g. clean drinking water, productive fisheries), or is it to see if a particular management action, like an environmental flow, has achieved what was intended? Is there an overarching vision for the future – i.e. what do you hope the river, or lake will be like in 10 or 50 years time? Can you explain what a ‘healthy’ system will be? For example: Will it have more fish? Will a population of endangered species be protected? Will the water be safe to drink or swim in? Thinking about these types of questions helps determine the indicators that you should consider and the thresholds of concern that you will need to set. With a clear vision statement, it is possible to identify the assets and values that reflect that vision, and the water quality and ecosystem health objectives that protect those values. Without a clear vision, a monitoring program will be poorly defined and unlikely to lead to improvements in ecological health. 2.2. Selecting appropriate indicators for the assessment As you will see from this review, there are many things that can be measured. It is better to select a few indicators that are meaningful and are known to respond to pollution and other threats than to try and measure everything. Indicators should be diagnostic and guide management actions, and also: • Quantify threats and assets (drivers, stressors and responses); • Provide easily interpretable outputs; • Respond predictably to damage caused by humans and at appropriate time scales; • Be cost effective to measure; • Relate to management goals; • Be scientifically defensible. There are many different threats to rivers and lakes and they often occur together (e.g. heavy metal pollution, agricultural runoff, flow alteration). Ideally, a monitoring program will be able to tease apart the effects of these different threats, so that appropriate management actions can be identified and prioritised. Developing simple conceptual models and diagrams (showing cause and effect) can help in this process by; • Showing how rivers and lakes function when they are healthy • Showing how the ecosystem will respond to pollution and other disturbances. • Identifying critical parts of the ecosystem to target for monitoring • Highlight appropriate management actions to address these problems The initial selection of potential indicators should be guided by their relevance to important environmental assets and values, and their likely response to different threatening processes. 2.3. Classifying water bodies into different types It is important to recognize the differences between types of water bodies when developing a monitoring program because: • • Lakes and rivers have different biotic assemblages and function in very different ways. Different types of rivers (and lakes) will not look and behave the same even when they are healthy. 10 • The types of indicators that might be appropriate in one type of river or lake may not be appropriate for another. • • The methods used to sample in one type may not be possible or relevant in another. Even where the same indicator can be used in different water body types, the thresholds or targets are likely to differ. Because of these natural differences, it is not appropriate to directly compare indicator values from very different types of water bodies. The way to address this problem is to develop a classification based on landscape and climatic features that are known to influence water quality and biota (such as rainfall, runoff, temperature, geology, topography and other landscape features), but are not influenced by human activity. • The scale of the classification will be determined by the spatial extent of the monitoring program. For example, a coarse classification would apply at the national scale and finer-scale classifications in specific regions. • Using the classification, it is possible to group together rivers and lakes and their catchments that are similar to one another • The selection of indicators and threshold values for indices can then be determined for each class of river or lake. Classification is an important step in ecological health assessment and river or lake management more generally, as it ensures that comparisons are made only between similar types of systems, and forms the basis for setting thresholds for determining what is in good health and what is not. 2.4. Testing the response indicators There needs to be confidence that the indicators you choose actually respond in a predictable way to stressors. This can be established by reviewing the literature to identify indicators that have been tried and proven in other places, or where possible, undertaking a field assessment pilot to test a range of potential indicators against a known disturbance gradient. For a field pilot study, it is important to select sample sites along a disturbance gradient. Land use information (using remote sensing or aerial photography and GIS) can be used to quantify catchment disturbance (see Box 1). Water resource use information may also be needed to quantify a disturbance gradient to hydrological regimes. Once sites have been selected along the disturbance gradient, all potential indicators are sampled over the same time period and using the same methods at each site. Data are analysed to select indicators that respond in a predictable way (e.g. linear or threshold change) to specific measures of disturbance. Indicators that are either too variable or show no clear trend along the disturbance gradient would generally be rejected. Some indicators may respond in a similar way to others. These ‘redundant’ indicators can be eliminated to reduce costs. At the end of this pilot stage, there should be a shortlist of suitable indicators that reflect the range of ecosystem health values of interest, and are known to be responsive to the range of threats that may occur. 2.5. Setting appropriate benchmarks and threshold values for the indices. To report on ecological health, it is important to set threshold or target values for each of the indicators (or indices) that reflect different levels of health. Most importantly, it is necessary to agree on levels that distinguish between ‘good’ (target or reference) and ‘bad’ (unacceptable) condition in a particular river or lake based on river or lake type (from the classification) and the specific management objectives. For example, different trigger values for water quality parameters are routinely set for different uses or values (e.g. drinking water, recreational contact, industrial use), and these are used to set thresholds for concern. For other environmental values (e.g. biodiversity targets), setting appropriate thresholds is not always as straightforward. It is often not feasible or desirable to take a ‘reference’ condition approach, comparing to systems that are undisturbed by human activities (see Box 2). By definition, if the indicator reaches a 11 level that does not protect or maintain the assets or values identified in step 1 above, then it would be considered to be a ‘fail’. The key point in setting targets for each indicator is that they must reflect both the type of river or lake and the management objectives and ultimately the desired vision for the river. 2.6. Reporting on the results The first question to consider is who is the report being prepared for and for what purpose? Different audiences (e.g. scientists, policy makers, general public) may require different levels of detail. Difference audiences may require different strategies for communication and engagement. It will be necessary to simplify the detailed and complex information from a suite of indicators into a simple score or report card. For example: • The threshold values for pass and fail may need to be rescaled (e.g. from 0 to 1) so that all indicators are comparable. • Individual indices can be combined within an indicator group (e.g. indices relating to fish can be combined as a single ‘fish’ score. Several water quality parameters can be grouped as an overall water quality indicator). • When combining indices, it may be necessary to take the minimum score rather than the average. For example, if the water is toxic for one metal, then it is regarded as toxic even if all other water quality indices are below their trigger value. • When combining indicator groups into a single report card score, different weightings may be given to some indicator groups depending on the goals of the program. . As well as reporting on the condition of individual sites at a particular point in time, it may be valuable to report on the proportion of sites in a region that are passing or failing, or to report on trends over time. Are most sites in good/bad condition or improving/degrading over time? This is a particularly useful approach in larger water bodies where information is collected from multiple sites. The way in which information is presented in a report card must make sense to the audience. It is primarily a communication tool and needs to be underpinned by a more technical report on the data. Box 1: Selecting the appropriate spatial and temporal scale When developing a river health assessment program, the appropriate scale required to define human disturbances must be determined, and selecting the appropriate measures of human disturbance is necessary. In many cases, the proportion of particular land uses or land cover types (e.g. residential land cover) in the drainage area of each site in a study region are used to represent a disturbance gradient against which site-based measures (or indicators) of river health are analyzed (see above). Values of indicators for any one site would be expected to reflect the amount of human disturbance associated with that site. However, sites along a particular large river may have similar proportions of land use in their upstream catchment areas, unless the sites are quite far apart (Angradi et al. 2009); therefore the use of alternate or multiple scales of land use/cover measures (e.g. sub-catchment, buffer zones, etc.) or other metrics and methods (e.g. distance to upstream urban area or incorporation of hydrological flow distance in land use metrics) may be needed (e.g. see Peterson, 2011). In a comparison of the ability of different land use/cover disturbance metrics to account for variation in fieldbased water quality and biotic indicators of stream health, Peterson et al. (2011) found that those based on inverse distance (= proximity) to the survey site tended to perform better for macroinvertebrate indicators, whereas those based on proximity to the stream were more suitable for fish indicators. In both cases, the inverse-distance-weighted (IDW) metrics out-performed the traditional, ‘lumped’ catchment metrics, which are non-spatial (e.g. % agricultural land in the entire catchment area). The IDW metrics of Peterson et al. (2011) used distance-decay functions to give more weight to land use closer to the survey site or the actual stream course (along its entire length, including tributaries, upstream from the survey site). The results of this study were based on ecosystem health assessments of streams rather than large systems, but the findings suggest that these alternate measures of human disturbance, which account for the spatial proximity and hydrological effects of land use, may also be useful in health assessments of large water bodies. 12 Some types of human disturbance, on land or in water, may act on local scales while others are likely to act on large scales or have cumulative effects on the ecological condition of rivers and large water bodies (Hunsaker and Levine 1995). Lammert and Allen (1999), found that local land use and habitat predicted biotic integrity, while regional land use showed no relationship. In contrast, Roth et al. (1996) in a study of the same catchment (but covering a larger area with greater contrast between sub-catchments) found that regional land use factors were relevant to biotic integrity. In some cases, localized disturbances may not have as widespread an impact on biota and ecological functions within very wide or deep water bodies as in narrow, shallow ones (cf. Angradi et al. 2009). In other cases, the effects of land use patterns on river health may be over-ridden by the effects of large point source disturbances often present in lowland reaches. In general, however, the total disturbance relevant to a particular river section will be the sum of both localscale and upstream factors (Blocksom and Johnson 2009), which can create spatial autocorrelation of human disturbance and of ecological condition among sites. The specific hydrological characteristics of a river system will also act across a range of spatial and temporal scales to interact with landscape-level and local environmental-scale features (disturbed or otherwise) to affect local assemblage characteristics, water quality and ecosystem processes (Leigh et al. 2010a). Thus, major hydrological developments (e.g. dams), often present in large river systems, are likely to affect a river’s physical, chemical and biological characteristics, as well as biotic-abiotic interactions, on both large and small spatial and temporal scales (Johnson et al. 1995; Blocksom and Flotemersch 2005). These studies all attest to the importance of defining and measuring human disturbances on spatial and temporal scales relevant to the ecosystem and/or ecosystem health indicator of interest. 2.7. Implementing the monitoring program to inform management. It is important to remember that monitoring and reporting is not the end of the process, and they need to be developed in the context of an adaptive management process that: • is clearly linked to identified values and objectives; • is informed by rigorous science; • guides management actions; • is responsive to changing perceptions and values of stakeholders. It is also very important to consider the resources (in terms of budget and staff) needed to develop and implement the monitoring program. In general the costs are tied to: • the number of field sample sites • how the field sites are selected • how frequently you sample in the field • the number of indicators you intend to measure • how frequently you intend to report Regardless of cost considerations, there are some aspects of the program that must be considered: • sufficient sites and measurements to achieve the statistical power required • a set of well written guidelines on how to carry out all aspects of the monitoring work • a quality control and quality assurance program to make sure the data are being properly collected and analysed • good training programs to certify the skills of the field and laboratory workers • ongoing technical review of the program to continually refine and improve the methods • a well resourced communications team whose job is to effectively communicate the results to management and the wider community, and to document the management responses • a data management system for archiving all of the data in a standard way, and for making the data and reports readily accessible Monitoring programs will not be effective unless there is strong collaboration and communication between policy makers and the scientists involved in undertaking the program. They will also fail if they are not sufficiently well planned and resourced. 13 Box 2: Defining reference condition for large water bodies ‘Reference’ is a term that has many definitions, but in the context of river health assessment, it generally refers to some benchmark against which ecological condition is assessed (Flotemersch et al. 2006b). Stoddard et al. (2006) provide four useful definitions of ‘reference condition’: minimally disturbed condition refers to that of rivers minimally impaired or unimpacted by anthropogenic disturbance; historic condition to the condition of the river at some point in the past; least disturbed condition to the best condition that exists currently; and best attainable condition to one that would be expected (e.g. modeled) of least disturbed sites or rivers under the best possible management practices. For an expanded discussion of these benchmarks and their relevance to conditions in China see Gippel (2010, pp. 7-11). Large rivers and lakes often flow through or are situated within intensely developed (agricultural and urban) catchments or at least have some history of anthropogenic disturbance (Weigel and Robertson 2007) such that traditional methods of health assessment based on comparisons of ‘reference’ and impaired sites may not be appropriate, particularly given that most sites in ‘reference condition’ are likely to be found in the upper catchment (on small streams) such that their comparison with lowland (large river) sites is questionable. Alternatives to that of the traditional ‘reference condition’ approach that have been used or tested in large water bodies include the stressor gradient approach (e.g. Angradi et al. 2009), the basic tenet of which being that within any river, there is a gradient of humaninduced disturbance from locations that are least-disturbed to those most-disturbed. Such ‘disturbance-gradients’ have been used to describe the responses of different biotic and abiotic indicators to anthropogenic disturbance in large rivers (e.g. Wessel et al. 2008) as well as streams (e.g. Bunn et al. 2010). Angradi et al. (2009) extended the approach by using regression models to define the ‘reference condition’ values of biological metrics when the disturbance was modeled to equal zero. Another option instead of using the traditional reference condition approach when dealing with large systems for which there is a long history of widespread and/or intense human disturbance, is that known as the ‘best attainable’ approach (where ‘best attainable’ is as defined by Stoddard et al. 2006). ‘Least disturbed’ sites in heavily disturbed systems may, in reality, be similar to those that are most disturbed (i.e. the gradient between least and most disturbed is minimal). Thus, any comparison between least disturbed (if used as a surrogate for ‘reference condition’) and disturbed sites may lead to ‘overly optimistic’ assessments of ecological condition (Wessel et al. 2008). In assessments of large water bodies that have moderate to high levels of human-induced disturbance, the ‘best attainable’ condition is thus a more useful concept when developing targets for indicators of ecological integrity. 14 3. Physical indicators of pressures 3.1. Hydrology Hydrology of large rivers Hydrology is an important driver of river health, and it is often impaired in large rivers due to their use for irrigation water supply, the presence of regulating dams upstream, the existence of water diversions (for town, industry and agricultural use), and the existence of weirs (for hydropower production, to allow convenient gravity diversion, or to create ponded water for its amenity value) (Poff et al. 1997; Bunn and Arthington, 2002). The principles of monitoring hydrology for river health assessment are the same regardless of the size of the river. Gippel et al. (2011a) considered the problem of how to assess hydrology in rivers for river health programs, with an emphasis on the conditions that apply in China. The calculation of general hydrological statistics is straightforward and convenient, being facilitated by the free availability of a number of computer programs, however, the results can be difficult to interpret in terms of river health impacts, and do not necessarily assist in deciding the most appropriate course of management action. Gippel et al. (2011a) proposed an index (IFD) that could be applied to historical monthly flow data, which comprises eight indicators, with each one having conceptual relevance to ecosystem health. The IFD highlights impacts of flow regulation, and also highlights years of naturally lower than usual flows, both of which are important determinants of ambient ecological health, as measured using bioassessment methods (Gippel et al. 2011a). At the very least, the IFD provides a simple way of establishing the relative hydrological health of rivers at the national and regional scales for gauging stations that have pre-regulation flow data available. The alternative suggested by Gippel et al. (2011a) for assessing hydrology was to first determine the environmental flow needs of the river, and then monitor the compliance of the flows in each year against these needs. This was considered the preferred method, but it requires considerable effort to establish the flow needs, and it also requires availability of daily flow data. Large rivers in China are often impacted by the presence of weirs. In rivers with many closely spaced weirs, the biggest change to the environment may not be the change in hydrology, but the change in hydraulics, with the river being converted to a series of weir pools with a stepped water surface profile and increased depth, reduced velocity, and reduced variation in depth and velocity along and across the river. Weirs are used to control water levels, such that variations in flow (across the range of low and moderate flows) may not give rise to significant changes in water level. Alternatively, if weirs are used for hydropower production, the regime is characterized by rapid discharge and water level fluctuations. In this situation, rate of rise and fall in water level will be an important hydrological indicator. Thus, in large rivers, proportion of the river that is effectively ‘ponded’ behind weirs, and the frequency and rates of water level fluctuations may be as telling as flow rates. Hydrology of lakes, wetlands and reservoirs The hydrology of lakes, wetlands and reservoirs is usually expressed in terms of the pattern of water levels through time – often referred to as the hydro-period. The important indicator is the duration and timing of the level of the water surface relative to certain ranges, with these water level ranges having some independently defined ecological relevance. For example, lake edge plant communities are often structured according to elevation (and distance from the water’s edge), which relates to how often and for how long they are inundated, or have access to groundwater. Sufficiently large water bodies can develop their own complex circulation patterns, driven by wind, water inflows, or thermal differences (e.g. Blukacz et al. 2009). Where the circulation pattern is critical in determining ecological health (such as occurs in water bodies that stratify) it may be necessary to 15 monitor the hydrodynamic conditions. Normally this would be done only in cases where the hydrodynamics were under management control. For example, it could be the case that inflows, outflows or water levels could be adjusted to achieve certain hydrodynamic conditions that were associated with known ecological outcomes (with this knowledge perhaps being an outcome of the health monitoring program). Remote sensing of hydrology While hydrology is generally measured at gauging stations, gauges are not always situated in the locations of interest. Also, in large lowland rivers the spread of water through floodplain wetlands is often of interest rather than the flow per se (e.g. Leigh et al. 2010a). Remote sensing of hydrology may allow these aspects to be incorporated into river health assessment programs. For example, Jung et al. (2010) characterized the different hydrodynamic flow patterns that occur in the Congo (Africa) and the Amazon (South America) Rivers during flood inundation using data from the Japanese Earth Resources Satellite. In the Brahmaputra River, Asia, they used in situ bathymetry measurements in combination with elevation and water slope data from the Shuttle Radar Topography Mission to estimate discharge with 2.3 percent accuracy. Jung et al. (2010) noted these types of analyses would soon be facilitated by the Surface Water and Ocean Topography (SWOT) satellite mission to provide hydraulic data (water surface elevation and slope) for rivers, lakes and reservoirs. However, in rivers with extensive levy banks or other structures that restrict overbank flows and floodplain inundation, these particular methods for characterizing floodplain inundation patterns may be less relevant to a river health assessment program. 3.2. Physical form Physical form is monitored in river health assessment principally because of its role in providing physical habitat for biota. Because of the predominance of material deposition (over sediment sourcing and transfer) in large rivers, backwaters, islands, woody snags and floodplain features assume greater importance for biota than in small-scale streams (Wilhelm et al. 2005). Physical form is also monitored because of concerns about sediment transport (silting reservoirs or channels), or channel migration threatening valuable assets (cultural, economic or natural assets). These concerns are often heightened in large water bodies and the ecological integrity of large river channels are often threatened by activities, structures and human-induced disturbances such as artificial channelization, dredging, shipping, dams and weirs, and bank erosion or artificial stabilization. Field-based assessment of river physical form Wilhelm et al. (2005) developed and tested a non-wadeable habitat index (NWHI) for large rivers, which included seven variables: • riparian width (measured from aerial photographs and in the field using a rangefinder); • large woody debris quantity (number of pieces); • aquatic vegetation (visually assessed percent cover in 20 x 10 m plots); • bottom deposition (visually assessed percentage of the bottom covered with soft muck, indicating deposition of fine silts); • bank stability (visually assessed severity); • thalweg substrate (visually assessed percentage gravel or larger); and • off-channel habitat [not specifically defined by Wilhelm et al, 2005), but understood to mean backwaters in the riparian zone, partially or fully connected to the main channel, which can act as refuge areas for biota]. Four of these variables (i.e. riparian width, large woody debris quantity, aquatic vegetation and bottom deposition) were included because of their statistical association with independently derived measures of human disturbance in the riparian zone and the catchment. Three (i.e. bank stability, thalweg substrate, and off-channel habitat) were poorly correlated with disturbance gradients but were included 16 because they are generally considered important in other habitat protocols or to the ecology of large rivers. The NWHI index was significantly correlated with indices of disturbance based on the riparian area and the catchment. Some of these measures of physical form, especially those reliant on visual assessment of physical form (see also Barbour et al. 1999), are likely to be of limited value in a river health program for China Gippel et al. (2011b), because they are subjective and unrepeatable. The recommendations made by Gippel et al. (2011b) apply to large rivers as well as small streams. They suggested variables that could be measured objectively to characterize provision of physical habitat, relative channel stability, and direct channel disturbances. These are of relevance to ecosystem health, and are also linked directly to potential management actions that could improve the physical form (if physical form is deemed to be limiting, or potentially limiting, ecosystem health). In general, as rivers become large, the majority of the ecological health-related variability in physical form shifts from small-scale variability associated with pool-riffle morphology and bed material size to plan-form variability (i.e. meandering, braided or anastamosing). Plan-form variability is easy to measure using remote sensing, while longitudinal bed variability and bed particle size generally require field measurement. The only way to directly monitor change in channel form is to survey the channel over time. Although this would seem like a straightforward, if expensive, procedure, this approach often suffers from technical difficulties to do with surveying, biased location of cross-sections, too few cross-sections to account for variability, and difficulties in extracting simple form metrics from the survey data. Even when repeated cross-section surveys are ostensibly undertaken at the same location, data supplied as x-y, or chainage-elevation files are not comparable. This is due to the impracticality of the surveyor being able to follow a dead-straight path across the river. Deviations from a straight line add distance to the crosssection; such that a particularly wandering or bowed survey path will give the impression that the channel is wider than it is in reality. Indirect monitoring of river channel stability Specific gauge analysis The functional relation between water level (also referred to as ‘stage’) and discharge at a river gauging station is known as a stage-discharge curve, stage-discharge rating or rating curve (DeGagnea et al. 1996). Standard hydrographic procedures are applied to the measurement of both stage and discharge, as well as the development of stage-discharge relations, and rating curves can be continually redefined through a regular program of manual flow gauging. A specific gauge plot is a time series of the water level (or stage) that corresponds to a given value of discharge. Usually the plot comprises a number of discharges that are selected to represent low, medium and high flow conditions. If the gauge site is hydraulically stable over time, then the plots are straight lines. Variations in stage for a given discharge indicate instability in the rating relation over time. regarded Gauge analysis is regarded as one of the most powerful tools to discern channel change (Blench 1969), and confirms that geomorphic re-equilibration of large rivers is a reach-scale process that can take decades or longer (e.g. Pinter and Heine 2005). Ideally, hydrometric gauging stations are located such that a stable stage-discharge relationship is achieved, and measurable changes in stage produce consistent changes in discharge (DeGagnea et al. 1996). In reality, stable sites are difficult to find, especially in lowland rivers. There are a number of reasons why a rating relation may not be constant over time; for example, because of backwater effects resulting from the growth of in-channel vegetation or build-up of debris, changes in approach velocities due to upstream hydraulic changes, or changes in channel morphological that affects the hydraulic control of the gauge site. Morphological change is a regular part of channel evolution, but its rate can be accelerated, or direction altered, by direct disturbance of the bed and banks, or through imposition of a regulated hydrological regime. Morphological change can also occur episodically in response to flood events. 17 A rising specific gauge plot potentially indicates that one or more of the following processes are active: • shallowing of the bed (aggradation, or sedimentation); • narrowing of the channel; • increasing roughness (due to build up of snags); • changing river operations that act to contain flows within the channel (such as raising the level of a downstream control structure, or closing regulators on effluent channels). A falling specific gauge plot potentially indicates that one or more of the following processes are active: • deepening of the bed (degradation, or scour); • widening of the channel; • decreasing roughness (due to removal of snags); • downstream meander cutoff; • changing river operations that act to release flows from within the channel (such as lowering the level of downstream control structures or opening regulators on effluent channels). These changes are only relevant if they impact the point of hydraulic control for the gauge site. This point may be located some distance downstream of the gauge, and its location may vary with discharge (Northwest Hydraulic Consultants Ltd 2003). For example, under low flow conditions, the hydraulic control might be a bed feature just downstream of the gauge, while under flood flow conditions the hydraulic control might be a valley-scale constriction hundreds of metres downstream of the gauge. The point of undertaking specific gauge analysis is to diagnose morphological channel change through time. In effect, the continual process of rating the gauge and adjusting the rating relation constitutes indirect monitoring of channel morphological change. One difficulty in interpreting a specific gauge plot is that a rise or fall in the relation can arise from a number of causes. It is normally possible to obtain independent information concerning river operations, meander cutoff, and de-snagging activities, which leaves the problem of distinguishing shallowing from narrowing, and deepening from widening. The main use of specific gauge plots is to draw attention to the possibility that a morphological change (instability) has occurred. Although specific gauge plots might provide clues to the most likely type of change that has occurred, they should not be expected to provide an unequivocal explanation for the cause of morphological change (Biedenharn and Watson 1997). Specific gauge analysis could provide important contextual information to a river health monitoring program because it has the potential to establish the existing geomorphological trajectory of the river. It could be that expectations regarding achievement of a stable river need to be framed within constraints set by an existing persistent trend in geomorphic trajectory. For example, some rivers are incised from previous vegetation clearance, and they continue to scour due to confinement of high flows within the channel; others are affected by oversupply of sediment from previous large-scale land disturbance. Such rivers may be unlikely to be expected to have a high level of health relative to ‘reference’, and improvements in health may be difficult to achieve. Specific gauge analysis can only be applied in situations where the gauge is situated on a river where the channel is free to adjust its morphology. This approach is unsuitable in rivers with hard-lined bed and banks, or where the gauge is at a weir. Stage height distribution analysis The susceptibility of river channels to fluvial scour is often assessed in terms of the shear stress or velocity to which they are exposed. Empirical relationships describe the likelihood of mobilization of bed material, or scour of bank material, to thresholds of shear stress or velocity. The threshold shear stress, known as the critical shear stress, is a physical property of the bed and banks. The critical shear stress depends on the particle size of non-cohesive grains, percentage content of cohesive silt-clay material, and extent and type of vegetative cover. Julian and Torres (2006) separated estimated bank shear stress into four properties: magnitude, duration, event peak, and variability. The event peak (maximum peak) of excess shear stress best predicted cohesive bank erosion where there was moderate critical shear stress, while the variability (all 18 peaks) of excess shear stress best predicted erosion when critical shear stress was low. However, the stream banks studied by Julian and Torres (2006) were not particularly high in silt-clay content. For streams with high silt-clay content (as is more typical of large rivers), the duration of excess shear stress was likely to be the most important variable (Julian and Torres 2006). Thus, large rivers that are used for conveying irrigation flows for long periods are subject to the risk of bank erosion, regardless of the natural cohesivity of the banks. In this situation, two related hydrological-hydraulic phenomena pose a risk to channel stability: • low variability in stage height (relative to the natural range), and • focusing of stage height over narrow bands (relative to the natural pattern). These two phenomena can be characterized using statistics that describe the variability and the peakedness of the distribution of river stage height. It would be straightforward to incorporate these indirect indictors of channel stability into a monitoring program using data that are already collected from river gauging stations. Physical form of lakes, wetlands and reservoirs One important characteristic of reservoirs on rivers that is often monitored is the rate of sedimentation. This is mainly of interest to dam operators but it has indirect relevance to ecological health via its affect on the life of the dam, and by imposing constraints on operation (which may affect of the health of the river downstream). Sedimentation in reservoirs may also be associated with nutrient concentrations and nutrient availability within the water column (depending on multiple factors including bioturbation and levels of oxygenation) and this can therefore affect both water quality and plankton dynamics, including those of algal blooms (see Assessment of biotic parameters). The rate of sedimentation can be measured either by sediment balance (measuring inflowing and outflowing sediment loads) or by bathymetric survey. Reservoirs on rivers with high sediment loads can experience growth of deltas at the points where rivers enter the reservoir. The growth of deltas can be monitored using standard ground and hydrographic survey techniques, or remote sensing. Large lakes and reservoirs do not necessarily have stable shorelines. This could be due growth of deltas (see above), or shoreline erosion. Alternatively, the physical form of the shoreline might not change, but its perimeter shape might change over time due to changes in the water level. Interspersion is the amount of interface between land (vegetated) and open water. Thus, a quantitative measure of interspersion would be the edge density, or perimeter length to lake surface area ratio (Mancini and Rusch 1988; Desgranges et al. 2006; Rehm and Baldassarre 2007). More complex-edged lakes have a higher edge density. The link between reservoir water quality and the physical characteristics of both reservoirs (e.g. shoreline complexity, water storage capacity) and catchments (e.g. land use and size) was demonstrated by Leigh et al. (2010b) in their study of 15 reservoirs across southeast Queensland, Australia. These physical factors together correlated strongly with nutrient and chlorophyll concentrations across the reservoirs as well as with densities of toxic cyanobacteria in summer months. Knoll et al. (2003) also linked lower reservoir volume to catchment area ratios with higher concentrations of chlorophyll a and total phosphorus in Ohio reservoirs, USA. In reservoirs with lower shoreline length to surface area ratios, the open water (pelagic) zone is likely to have a stronger affect on water quality than that of the shore edge (littoral zone), which may exacerbate water quality issues in eutrophic systems (Leigh et al. 2010b). Mudflat habitat may also be important to assess in terms of the ecological health of large waterbodies. This habitat type is important to certain waterbird species that inhabit lowland rivers, wetlands and lakes (Crome 1988, Scott 1997). Seasonal mudflat exposure, in response to falling river or wetland levels, ensures mudflat resources are available to waterbirds. With shallower water the fish community 19 becomes more susceptible to avian predation. A community of invertebrate macrofauna exists within and on mudflats, providing the basis of a complex and productive food web (Baxter et al. 2005). There are two ways to monitor edge density and mudflat availability through time. One way is to obtain aerial or satellite images of the water body, and the other is to survey the water body form (bathymetry and topography) and then monitor the change in perimeter shape over time as a function of changing water level. Remote sensing of physical form A remote sensing approach to monitoring physical form allows consideration of catchment-wide stream networks or entire reaches. This is in contrast to field-based approaches that are limited to sampling a limited number of sites over a relatively short length of channel and floodplain. Physical form varies across a number of scales, and the largest of these cannot feasibly be sampled in the field. Channel form is fundamental to geomorphological characterization of rivers. The standard approach is on-ground surveys that incorporate hydrographic survey (using boats with sounders). The remote sensing alternative is LiDAR (Light Detection And Ranging). Use of LiDAR to obtain channel morphology data is now routine (Bowen and Waltermire, 2002), and progress has been made in automated extraction of relevant data (e.g. Miller et al. 2004). Standard airborne LiDAR does not penetrate water, so any rivers and wetlands that contained water at the time of the flight would require supplementary hydrographic survey. More advanced airborne LiDAR bathymetry (ALB) systems (using a combination of green and blue bands) can penetrate water. Under ideal conditions in oceans, penetration of 50 m is possible. However, in rivers, overhanging trees, aquatic vegetation and entrained air will cause interference. Even without these problems, penetration is severely limited by turbidity, which scatters light, and dissolved organic material, which absorbs light. Penetration is around 2 – 3 times the Secchi depth (Hilldale and Raff 2007). Secchi depth of a clear water body would be >2 m, while for low to moderately turbid water bodies the Secchi depth would be 0.5 – 1.0 m or less. This means that blue/green band LiDAR may not penetrate the full depth of some water bodies. Remote sensing approaches have been used to show the relationships between geomorphic complexity (e.g. having expansive, multichanneled floodplains and/or on-channel lakes) and fisheries productivity (e.g. salmon, Luck et al. (2010). They can also be used to distinguish classes of floodplain vegetation that correspond to successional age and reflect the activity of riverine meanders (Hamilton et al. 2007), and to map in-stream habitats (e.g. Whited et al. 2002, Leckie et al. 2005). 20 4. Water quality indicators The physical and chemical characteristics of water can be used as direct measures of health (e.g. to meet human drinking water or amenity values), as well as indicators of pressures on other ecological values (Bunn et al. 2010). Given the cumulative impacts of human activity in upstream catchments and tributaries and the additional threats from city and industrial effluent, a broad range of water quality indicators will be particularly relevant to large water bodies. Nutrients from upstream agriculture and point source discharge from city effluent, causes algal blooms and the loss of amenity and recreational values, as well as threats to drinking water security. It may be important to identify which nutrients (e.g. N or P) are most important in promoting excessive algal growth and whether they are derived from local point sources or diffuse sources from upstream. Turbidity from diffuse sources upstream and compounded by urban run-off and turbulence from dredging and shipping will impact on light regimes and influence the growth of algae and other aquatic plants. Organic matter loading from sewage treatment plants and chemical loading from some industries will have a major impact on the availability of oxygen and in turn impact on the distribution of plants and animals. Toxic metals and other chemicals from industry and urban storm-water pose additional problems for larger water bodies, as does the thermal pollution from dams or power plants. 4.1. Field sampling methods The methods for assessment of water quality are well established and common examples used in assessments of large river and lake condition are briefly described below. Smith and Tran (2011) used 1 m depth integrated, surface water samples from large river sites across New York State, USA, to determine total nitrogen and phosphorus concentrations. They collected two samples at each site and used the mean of the duplicate samples to indicate nutrient concentrations. Water chemistry data were also used by Weigel and Robertson (2007) for their assessment of nonwadeable rivers in Wisconsin, USA. For nutrient concentration data, they collected monthly water samples from each site using a hand-held sampler when rivers were wadeable, and using a cablesuspended sampler when the site was too deep or water velocity too high to collect samples by hand. Specific water conductance (electrical conductivity), dissolved oxygen, pH, and water temperature were also measured in situ using multiparameter data sondes, along with water clarity as measured with a 120 cm transparency-Secchi tube. The authors used the median values of the six monthly water samples from each site in their statistical analyses. A different sampling method was used by Blocksom and Johnson (2009), whereby in situ measures of water physico-chemistry were taken at the most downstream transect (out of 6) of each their 500 m long sampling reaches. These samples were most likely taken from the middle of each downstream transect, following Herlihy and Hendricks (2000). In lakes and reservoirs, replicate depth-integrated (e.g. using a van Dorn sampler) samples of surface and bottom water are often taken at multiple sites along a longitudinal (upstream or inflow zone, middlelake region, and near the dam wall or at the outflow zone) and/or lateral (e.g. shore and open water zones) axis by boat (e.g. De Ceballos et al. 1998; Leigh et al. 2010b). Samples are typically analyzed for concentrations of organic and inorganic nutrient, suspended solids and chlorophyll, as well as for phyto- and zoo-plankton densities and taxonomic richness. Sediment samples may also be collected (e.g. using a sediment corer) to examine sediment nutrient concentrations, as well as diatom assemblages (see Assessment of biotic parameters).Vertical profiles of light irradiance, water temperature, turbidity, conductivity, dissolved oxygen, pH and fluorescence are also commonly measured using multi-parameter sondes lowered from the boat via hand-held or winch-operated cables. These profiles may be used to establish whether the water column was stratified at the time of sampling, and can also provide insight as to the potential availability of nutrients in bottom waters to plankton that are not restricted to the euphotic zone, as well as the potential distribution of available habitat for oxygen depended biota such as fish and benthic macroinvertebrates. Baker et al. (1997) provide a 21 summary of techniques developed to assess lake ecosystem condition in the USA, including methods for detailing physical and chemical characteristics of water. It is important to note that water samples or measures must be taken at the spatial and temporal scale relevant to the assessment. For example, electrical conductivity and suspended sediment loads during high flow events are unlikely to provide suitable snap-shots of river health unless characterization of high flow events is the specific purview of the assessment program in question (see the continuous monitoring section below). 4.2. Continuous monitoring Probes for continuous monitoring of electrical conductivity, temperature and turbidity are now standard tools used to monitor streams, lakes and rivers. Due to their spatial permanency and/or cost, these are often used to provide high frequency (i.e. high temporal-resolution) data rather than high-spatial resolution data. This may have application in health assessments that aim to consider the site-specific loading of sediments, for example, into a river system, reservoir or lake during low, average or (in particular) high flow events. Continuous monitoring of temperature and dissolved oxygen over 24 h periods (e.g. every 15 min) is also commonly employed by river health assessment programs in order to capture the natural diel variation in these parameters, and therefore to account for this variation when assessing river condition. Continuous monitoring of these two parameters, along with discharge, has also been used to calculate ecosystem metabolism in streams (e.g. Izagirre et al. 2008) and may be of benefit in large water body health assessments, where in situ (chamber-based) methods have also shown promise (Fellows et al. 2007). 4.3. Remote sensing Satellite remote sensing and aerial imaging technology have long been used to monitor water quality of lakes and large rivers (principally turbidity, suspended solids concentration, chlorophyll a and phosphorous), and water temperature (e.g. Baban 1993; Tassan 1997; Zilioli and Brivio 1997; Oestlund et al. 2001; Senay et al. 2001; Kloiber et al. 2002; Shafique et al. 2003; Tyler et al. 2006; Nechad et al. 2010). Shafique et al. (2003) demonstrated that the hyperspectral remote sensing technique can be a useful tool for monitoring distributions of chlorophyll a concentrations in large rivers. In this study in Ohio, USA, the wavelengths of 675 nm and 705 nm from CASI (Compact Airborne Spectrographic Imager) data were found to be the most suitable wavelengths for predicting chlorophyll a concentrations. Chlorophyll a has a unique spectral signature and it is possible to estimate chlorophyll a concentrations for any inland water body with the chlorophyll a spectral index. Phosphorous and turbidity were also successfully monitored by Shafique et al. (2003), but these parameters lack a unique signature and would require calibration for each river or region. Martinez et al. (2007) used MODIS (Moderate Resolution Imaging Spectroradiometer) images to monitor inter-annual variation of sediment load by comparing surface reflectance data and field measurements of suspended sediment concentration collected every 10 days in surface waters of different locations of the Amazon River in Brazil. A similar study by Wang et al. (2010) on the Middle and Lower Yangtze River, China, found that the water reflectance difference between MODIS Bands 2 and 5 provided a relatively accurate representation of suspended solids concentration. Lower errors were achieved at higher concentrations of suspended solids. The same authors (Wang et al. 2009) investigated the use of Landsat ETM+ (Enhanced Thematic Mapper) to monitor suspended solids concentration on the Yangtze River. A regression relation between suspended solids concentration and water reflectance of Band 4 provided a relatively accurate estimate for sediment concentrations in the -1 range of 22 – 2610 mg L . 22 5. Biotic indicators Biota have been used to assess the ecological condition of large water bodies across the globe, for example in North America (e.g. Flotemersch et al. 2006) and Europe (e.g. Noble et al. 2007) and more recently in China (however these assessments were based on methods traditionally applied in smaller systems, see Bond et al. 2011; Leigh et al. 2011). There are many factors that must be considered in relation to sampling methods for biota in freshwater systems, including large water bodies. For example, as rivers become wider and deeper, the open water habitat generally becomes more important to biota and this part of the river may require different sampling methods than those used in benthic and littoral zones e.g. electrofishing versus nets for fish sampling, dip nets versus drift nets or grab samplers for macroinvertebrate sampling (Flotemersch et al. 2011). If safety, practicality or costs prohibit the use of boats, for example, to collect biotic samples from deep water habitats, then sampling procedures may need to be confined to wadeable sections. This should not invalidate the assessment, but will place restrictions upon it, noting that the particulars of any sampling regime and study design should be taken into account when analyzing data and drawing conclusions from the results (e.g. Parsons and Norris 1996). For example, if macroinvertebrates have only been sampled from wadeable, slack-water zones with aquatic macrophytes, then conclusions should only be made in the context of those habitats and the fauna expected to occur within them. An assessment program must therefore consider study design, sampling effort, the appropriate sampling techniques required to sample the range of habitat types available (or of interest) in large water bodies (Blocksom and Flotemersch 2005) and, when large numbers of organisms are collected, the appropriate laboratory subsample sizes to use (Flotemersch et al. 2006a). Several studies have been conducted recently on reach selection and sampling effort required for field-based bioassessment of large rivers. These are discussed below together with a review of the literature examining sampling and laboratory methods explicitly for specific groups of biota. Downes (2010) also provides a thorough discussion on random sampling of reaches, sites and habitats, on replication and on compositing and subsampling of biotic samples to detect effects of human impacts. 5.1. Diatoms and other algae In rivers, the use of diatom-based indices of water quality has increased rapidly in Europe and elsewhere, following on from Whitton et al. (1991) and Kelly and Whitton (1995), with one of the earlier large river bioassessments to use diatoms being that of Fore and Graff (2002). More recent discussions of their use in large river bioassessment include that of Reavie et al. (2010). Four methods of sampling diatoms from large rivers in the Ohio River basin, USA, were compared by Lane et al. (2007) on the basis of sampling effort and response to disturbance gradients. The methods consisted of three that sampled periphyton from the littoral zone using multiple collections from multiple substrata, and one method that collected phytoplankton using three grab samples of water (Table 1). All samples were preserved in formalin, from which diatom valves were cleaned and mounted. From each sample, at least 500 values were identified. Diatom assemblage composition was similar among samples collected by all methods; however, the phytoplankton method appeared to collect a significant proportion of unique taxa compared with the littoral zone methods. Although the metrics calculated from all four methods appeared to show a response to the eutrophication gradient identified in the study, the phytoplankton method required the least sampling effort and was considered a suitable alternative to the more fieldintense periphyton sampling methods. In lakes and reservoirs, water samples (for phytoplankton) and sediment samples (for diatoms), along with other water quality parameters are often collected as part of regular monitoring programs or longterm research projects. These data may be suitable to assess ecosystem health through time (e.g. Moiseenko et al. 2006). Alternatively, sediment samples may be used to assess lake condition and historical changes in water quality. It has been shown that lake diatom assemblages are strongly 23 associated with gradients in environmental factors (e.g. pH and nutrients) (Dixit et al. 1999). Based on these relationships, in a study of 257 lakes and reservoirs in north-eastern USA, Dixit et al. (1999) examined diatom assemblages from the ‘top’ (surface sediments, present-day) and ‘bottom’ (generally from >30 cm deep, representing historical conditions) layers of sediment core samples to infer ecological condition and anthropogenic impact. O’ Connor et al. (2000) conducted a similar assessment of 19 lakes across northeast USA based on sediment core samples from the deepest point of each lake. Baker et al. (1997) recommend that 500 individual cells should be identified and counted from each core and, to ensure cores represent the pre-industrial to contemporary time period, the cores should be at least 45 cm in depth, when lakes have Secchi depths of 2.5 m or less, and at least 35 cm otherwise. These depths were recommended for lakes in the USA, and as such, may need adjustment for other regions. Baker et al. (1997) also recommended that when lakes are artificial, the core profile should not include the soil profile below the sediments. Contemporary assessments of lake and reservoir health may be similarly based on phytoplankton and sediment diatom sampling. For example, in a rapid assessment of 15 drinking-water reservoirs in southeast Queensland, Australia, Leigh et al. (2010b) collected a 3 m depth-integrated surface water sample from each of three sites in each reservoir (located at the most upstream end of the reservoir that was at least 6 m deep, the middle-reservoir region, and near the dam wall, each at the thalweg of the dammed river). From each sample, a 500 mL sub-sample was preserved with Lugol’s iodine solution to a final concentration of 0.6%, from which phytoplankton taxa were identified. In very large reservoirs, or those that dam the flow of more than one major river, it may be necessary to collect samples from more than three sites (see Leigh et al. 2010b). 24 Table 1: Periphyton sampling methods investigated by Lane et al. (2007). Method (code) Description Distance (m) Banks Method Quantitative Periphyton (EMAP) Composite samples collected from erosional (rock and wood substrata) and depositional (soft sediment) habitats such that a total of 2 132 cm of substrata were sampled at each stream reach 11 evenly spaced transects along a 2000 m reach (maximum) One Periphyton dislodged from a 2 12 cm area on upper surface of rock/wood using a stiff-bristled toothbrush for 30 s, then washed into a 500 mL bottle using stream water. For soft sediment, the top 2 1 cm of a 12 cm area was vacuumed into a 60 mL syringe Quantitative Periphyton (QUAN) Composite samples collected from surfaces of natural substrata in the expected richest habitat in the sampling reach, as identified following the procedures of Porter et al. (1993) 5 locations within a 1000 m sampling reach Both Periphyton collected from 5 ‘representative’ substrata at each location to give 25 samples composited into a single sample 2 that represented ~133 cm of substratum Qualitative Periphyton (QUAL) Composite samples collected from all instream microhabitat types present in the same sampling reach as that used for the QUAN method 5 locations within a 1000 m sampling reach Both Equal volumes of multi-habitat samples from all microhabitats composited into a single sample Qualitative Phytoplankton (PHYTO) Composited samples collected from the euphotic zone using a boat 3 locations equally spaced across the river n/a At locations where the euphotic depth > 1 m, each of the three composite samples was depthintegrated (top, middle and bottom) using a 1 L Van Dorn sampler. For shallow euphotic zones, a 1 L wide-mouthed bottle was used to collect grab samples, while facing upstream, from the top half of the water column at each location Table notes: Table based on information presented in Lane et al. (2007). Methods were based on the Environmental Monitoring and Assessment Program (EMAP) for non-wadeable streams and rivers of the U.S. Environmental Protection Agency, based on Hill and Herlihy (2000), and the National Water Quality Assessment Program (NAWQA) of the U.S. Geological Survey, based on Porter et al. (1993). 25 5.2. Macroinvertebrates Blocksom and Flotemersch (2005) looked at existing methods of macroinvertebrate-based assessment of river condition in the USA (Table 2), specifically to assess differences among sampling methods in terms of the assemblages collected and in the ability of the derived metrics to detect anthropogenic impacts. The study assessed impacts within a 500 m wide riparian corridor either side of a sampled river for a distance of 4 km upstream from the centre of the sampled reach. The drift net method (Table 2) did not collect macroinvertebrates effectively. All other methods were effective but taxonomic composition differed among samples collected using different methods. In addition, metrics calculated from the different methods were not consistent in their correlation with abiotic (disturbance gradient) measures. The authors concluded that the methods were not interchangeable and had different capacities to detect different types of stressors. They recommended that macroinvertebrate bioassessment methods use a combination of systematic, semi-quantitative methods (like the EMAP-SW kick net method, which helps to ensure that habitats unobserved by the human eye will be sampled) and more qualitative methods (like the Ohio EPA 500 m multi-habitat method, so that taxa missed by the particular positioning of the kick net are also collected) (see Table 2). In their study on the effects of sampling design on macroinvertebrate-based metrics used in large river bioassessment, Flotemersch et al. (2006a) aimed to determine the appropriate size of laboratory subsamples of field-collected macroinvertebrate samples. They recommended that a fixed laboratory subsample size of 300 organisms was suitable for bioassessment purposes, as opposed to more rigorous studies, which may require 500 organisms. The effect of mesh-size on the description of macroinvertebrate assemblages in large rivers was examined by Battle et al. (2007). They found that finer mesh (355 or 500 µm compared with coarser mesh >1000 µm) produced a more accurate estimate of assemblage structure and density and suggested that comparisons of assessments based on different mesh-sizes could be problematic. Another approach that has been used in macroinvertebrate-based assessment of non-wadeable rivers in the USA was to sample all available habitats at each transect, e.g. fine particulate organic matter, sand and coarser substrates, large woody debris (snags) and macrophytes, with a D-frame dipnet (500 µm mesh) for 15 seconds at each habitat (Wessel et al. 2008). Habitat-specific macroinvertebrates samples were then preserved in the field and identified to family level before combining the samples and using one-quarter of the composited habitat samples (excluding rare taxa) for analysis. Following on from the transect and kicknet-multihabitat approaches of Blocksom and Flotemersch (2005) and Flotemersch et al (2006a), Blocksom and Johnson (2009) modified the macroinvertebrate sampling methods used in large river bioassessment for the Midwest USA, primarily to increase standardization in the field. Macroinvertebrates were collected using a 0.3 m wide D-frame dipnet (500 µm mesh) by performing six 0.5 m long sweeps within a 10 m zone of each transect (5 m either side) that extended to the river’s midpoint or until the water was deeper than 1 m. When water depth at the edge was > 1 m, the sweeps were collected along the bank from a boat. The six sweeps were divided proportionally among the available habitat types (e.g. macrophytes, sand, gravel, snags etc.) at each transect. All samples from a reach (six 0.3 m x 0.5 m sweeps per sampling zone at each bank on each of 6 transects) were combined into one sample. In the laboratory, macroinvertebrates were sorted and identified from each sample from randomly selected grid squares on a gridded pan until the total count per sample was within 300 organisms. There are few examples of benthic macroinvertebrate-based assessments of ecological health in lakes and reservoirs. O’Connor et al. (2000) collected macroinvertebrates from 19 lakes across northeast USA using a petit Ponar dredge at the deepest point of each lake as well as timed dip net sweeps within the littoral area. Baker et al. (1997) recommended a more detailed method for lakes and reservoirs in the USA, whereby invertebrates are identified from sediment core samples collected from soft, weedless sublittoral areas from similar depths at 10 evenly spaced, randomly selected, sites around each lake’s perimeter. The water depth recommended at each sampling point depended on whether the lake was 26 stratified: in stratified lakes, the samples were recommended to be collected in well-oxygenated areas at depths “equal to or less than the depth where the upper limits of the metalimnion intersect the lake bottom”; in non-stratified lakes, the depth was recommended to be > 1 m. Baker et al. (1997) further recommended that invertebrates should only be identified from the top 13 cm of the cores, and for each lake a composite sample of every second core from the perimeter should be made, divided into eight equal parts from which invertebrates are identified until 150 individuals (excluding “microcrustaceans, plankton, nematodes, terrestrial insects, dead or empty snail shells, and all other nonbenthic animals that may have settled on the bottom of the lake”) have been counted. Table 2: Macroinvertebrate sampling methods investigated by Blocksom and Flotemersch (2005). Method (program) Description Distance (m) Banks Mesh size (µm) Drift net (EMAP-SW) Two drift nets set for 3–4 h during daylight hours at lower end of reach, in water of adequate depth Two locations Either or midreach 595 Kick net (EMAP-SW) Two 20 s kicks at each transect 11 transects over 2000 m One 595 500 m qualitative multi-habitat (Ohio EPA) All available habitats with D-frame net and hand-picking, minimum 30 min 500 m Both 595 Hester-Dendy multiplate substrate samplers (Ohio EPA) Five multi-plate samplers attached to a single block in lower 500 m of reach Single location One 595 1000 m qualitative multi-habitat (NAWQA) All available habitats with D-frame net and hand picking, approximately 60 min 1000 m Both 210 Richest targeted habitat (NAWQA) Five to six sites of the richest targeted habitats (according to prioritized list) 2000 m Both 425 Table notes: Table modified from Blocksom and Flotemersch (2005). Methods were from the Environmental Monitoring and Assessment Program for Surface Waters (EMAP-SW) of the U.S. Environmental Protection Agency, the National Water Quality Assessment Program (NAWQA) of the U.S. Geological Survey and the Biological and Water Quality Monitoring and Assessment Program of the Ohio Environmental Protection Agency (Ohio EPA) Division of Surface Water. 5.3. Fish Several methods are available for sampling fish in rivers, including seine netting, hoop or fyke netting, and electrofishing, and the selection and manner in which these methods are used can depend on the conductivity and turbidity of the water, water velocity and depth, all of which may also determine fish assemblages due to habitat preferences. Each method also tends to have particular sampling bias, with some methods (e.g. seine nets) selecting for smaller bodied, slow-moving fish while others (e.g. hoop nets or electrofishing) may select for larger-bodied fish (Li and Li 1996). Utrup and Fisher (2006) compared seine (6.1 m 1.2 m 4.8 mm mesh) and hoop (0.9 m 3.7 m 50.8 mm mesh, and 0.6 m 2.4 m 25.4 mm mesh) netting at 15 sites across five large rivers in 27 Oklahoma, USA, to determine sampling effort required to detect maximum species richness in each habitat and the selectivity of fish species that each method detected. At each site they sampled 11 transects, 100 m apart, in which they defined the different fish habitat types by water depth (shallow, < 0.75 m, or deep, > 0.75 m) and velocity (slow, < 0.20 m/s, or fast, > 0.20 m/s) or as non-wadeable (> 1.50 m deep) or backwater habitats. In each 1000 m reach, they seined twice in four randomly chosen shallow habitats and all backwater habitats, hauling the seine parallel to the shoreline for 10 m with the current. In deep and non-wadeable habitats they set six large and six small, unbaited hoop nets overnight for 12 h. For the studied rivers, the authors found two distinct habitat types based on species composition (shallow backwater and deep non-wadeable habitats) and that each site required 6-10 and 1-6 of each type, respectively, to be sampled in order to capture the fish assemblage effectively, and that this required a non-consistent sampling distance of 400-1600 m (i.e. distance was determined by the habitat characteristics of each site). Although the seine netting captured more species per unit effort, the hoop netting tended to capture larger fish. In a later study by Neebling and Quist (2011), electrofishing, seining and trawling methods were compared in non-wadeable rivers in Iowa, USA. Electrofishing captured the most species, and only fifteen 100 m passes were required to sample 90% of the species when reach lengths were 3 km as compared with 25 passes for 5 km reaches. Thus, the specifics of method selection, sampling effort, sample length and sampling location for fish may depend on the characteristics of the river in question and whether particular fish species or all fish species are targeted by the assessment (see above). Fish have also been used in bioassessments of lakes and reservoirs. For example, in their assessment of three regions of two Great Lakes in North America (Lake Ontario and Georgian Bay), Minns et al. (1994) electrofished 188 m transects in the littoral zones of each region. The transects ran parallel to the lake shores, approximately following the 1.5 m depth contour, and covered the range of near-shore habitat types available in each region. However, just as with fish sampling in rivers, the most appropriate sampling gear and effort required for fish surveys in lake or reservoir bioassessments may depend on the particulars (physical characteristics, habitat types, water quality etc.) of the system of interest. For example, Blocksom et al. (2009) found night-time electrofishing along 500 m lengths at 15 sites within each of seven major navigational pools (dammed river sections) along the Ohio River, USA, was sufficient to capture approximately 90% of the fish assemblage within each pool. It is important to note that commercial fisheries data may be available for some larger rivers and lakes and may be used to develop appropriate indicators of the health of this important group. 5.4. Vegetation Field sampling Riparian and in-stream vegetation were sampled as part of two pilot studies for large river health assessments in northeast (Leigh et al. 2011) and southeast China (Bond et al. 2011). In both studies, riparian vegetation was surveyed in three 50 m sections within a 500 – 1000 m reach at each site. Measurements from the three sections were then averaged across each reach to give a single value for each site. In each section, and on both river banks, riparian vegetation width was measured using a rangefinder, continuity of vegetation was scored (0 = no vegetation, 1 = isolated/scattered, 2 = regularly spaced, 3 = occasional clumps, 4 = semi-continuous, 5 = continuous), and tree, shrub and herb cover was estimated. Mean and maximum tree height and the number of saplings present in each section were also recorded, and the dominant tree species was noted. Instream vegetation (aquatic and semiaquatic macrophytes) cover in each section was also estimated, split into three broad categories of submerged, floating or emergent macrophytes. However, floating macrophyte cover (especially of invasive or nuisance species) was given particular recommendation above the other measures of instream vegetation. In addition, the authors of these two pilot studies noted that growth of instream macrophytes varies substantively throughout the year, with maximum production occurring in the warmer months, such that temporal consistency of sampling (or at least awareness of this growth cycle) would be required of any river health assessment program. 28 Remote sensing The launch of Landsat in the early 1970s offered the prospect of remotely sensing land cover. Satellite remote sensing and aerial imaging technology has been used for characterizing riparian, littoral and open water vegetation cover and condition for at least 25 years (e.g. Raitala et al. 1985; Narulamani et al. 1997; Muller 1997; Malthus and George 1997; Weberm and Dunno 2001; Congalton et al. 2002; Malthus and Karpouzli 2003; Valta-Hulkkonen et al. 2003; Maheu-Giroux and Blois 2005; Valley et al. 2005; Goetz 2006; Nelson et al. 2006; Shanmugam et al. 2006; Ashraf et al. 2007; Johansen et al. 2007; Booth et al. 2007; Yang 2007). Field-based mapping of vegetation cover is labor intensive, limited by accessibility, and more expensive than collecting data using remote sensing (Nelson et al. 2006; Ashraf et al. 2007). For vegetation monitoring, field-based data needs to be collected so that accurate spectral signatures of different vegetation types can be obtained and used to train the classification algorithms (Ashraf et al. 2007). Some key issues to consider are resolution of the imagery, timing of the images (to match seasonality of the vegetation) and cost of the imagery (which is higher for current as opposed to archived images, high resolution as opposed to low resolution images, and local- as opposed to regional- and national-scale programs) (Ashraf et al. 2007). 5.5. Ecosystem processes Functional indicators of river and stream health, including those that represent ecosystem processes, are increasingly being considered for inclusion in river health assessment programs (Bunn et al. 1999). Leaf breakdown rates and ecosystem metabolism have both been suggested as useful measures of functional river health (Young et al. 2008). However, as these measures can depend on channel width and position within the river system (e.g. headwaters, middle reaches, and lower floodplain reaches), condition and extent of riparian vegetation (which provides both shading and leaf litter to the river, and can also vary naturally from headwaters to lowlands), water temperature and seasonality; these factors must all be considered in the study design for health assessments of large water bodies. Continuous monitoring of dissolved oxygen and temperature, along with discharge, has also been used to calculate ecosystem metabolism (primary production and respiration) in streams (e.g. Izagirre et al. 2008) and may be of benefit in large water body health assessments, where in situ (chamber-based) methods have also shown promise (Fellows et al. 2007). Stream metabolism is sensitive to human-induced disturbance (stressors) including riparian-zone modification, siltation, eutrophication and hydrologic alteration (often key threats to large river systems) and is generally a low-cost method of assessment. 5.6. Sampling effort and study design in field-based bioassessments Large rivers There are many factors to consider when designing field studies in the context of large river health assessment. Firstly, the positioning of study reaches may be important to establish, as factors such as stream confluences, roads and obvious sources of impact, e.g. major dams, within the reach and/or sampling region are likely to complicate data analysis and confound results (Flotemersch et al 2006a). However, ensuring random selection of sites, reaches and rivers (i.e. location) has important consequences for identifying causality between stressors and ecological responses (Downes 2010). Along with reach selection, other factors to be considered include: the appropriate number of sampling points needed along a river or reach to assess condition; and, for assessments based on biota, the effects of reach length on assemblage characteristics (Flotemersch et al. 2011). In small stream and river assessments, reaches (as geomorphic units) are often used as sites because they are commonly less than one or two hundred metres long. However, in large rivers, a reach may span hundreds or thousands of metres and require greater sampling effort to collect resident biota (Flotemersch et al. 2011). There is a dilemma that sampling large sites may obscure localized changes in habitat, while sampling at smaller scales may restrict assessment of large scale impacts (Flotemersch et al. 2011). 29 Comparisons of macroinvertebrate sampling effort on functional and taxonomic diversity measures were made by Bady et al. (2005) based on quantitative data collected from three large rivers (width > 40 m) in Europe: the River Danube, River Rhine and Loire River. The study found that 10 replicate samples provided insufficient estimates of taxonomic richness, which was also dependent on season and site location. However, less sampling effort was needed to give the same level of accuracy for functional (biological trait) diversity measures as for taxonomic measures. Flotemersch et al. (2006a) collected macroinvertebrates from the Ohio River basin, USA, to investigate how many sampling points were needed for bioassessment in non-wadeable tributaries, how reach length affected the assemblages and to determine an appropriate laboratory subsample size. They presented a sampling protocol that can be used in the form of a pilot study to determine the most appropriate sampling design for macroinvertebrate bioassessment of non-wadeable rivers. They used a combined kick-net and qualitative multi-habitat sampling method, based on the findings of Blocksom and Flotemersch (2005), to sample both banks at 12 transects separated by progressively greater distances within six reaches in each of two rivers, from which macroinvertebrate metrics were calculated (including richness and proportional richness of all taxa and particular taxonomic or functional groups). Reach length was dictated by mean wetted width of the channel, being 40 times that of the wetted width (based on assessments of fish assemblages). The authors concluded that a ‘representative’ sample of the assemblage was attained by sampling both banks on 6 transects, and that consistent relationships between reach length and metric values suggested that study reach length could be determined by the spatial scale (e.g. repeating geomorphic units) in question. However, for standardization purposes, they suggested that transects be separated by 100 m across a study reach defined by a total river length of 500 m. Wessel et al. (2008) used macroinvertebrates to assess large rivers in Michigan, USA, again based on a sample reach length of approximately 40 times that of the channel width (which for the studied rivers had a mean of 89 m). However, they standardized the US EPA-recommended proportional channelwidth to reach-length approach (Lazorchak et al. 2000) by setting reach length at 2000 m, with each reach then divided into 11 evenly spaced transects (i.e. a transect every 200 m). For adequate characterization of fish assemblages in non-wadeable rivers, Meador (2005) compared the effectiveness of single-pass versus two-pass boat electrofishing. The comparison was based on two-pass data collected from multiple river reaches, 500-1000 m in length, across the USA. The author concluded that when true species richness was ‘low’, the single-pass method may be effective, but twopasses would generally produce a better estimate. In addition, relationships between reach length to channel-width ratios and species richness estimates were not able to provide a clear recommendation on the most effective sampling distance for the passes. However, the greater the distance sampled (e.g. > 1000 m), the better the estimate is likely to be from a single pass, and Meador (2005) recommended that factors such as species patchiness, habitat preferences and channel modifications should be taken into account if the single-pass method was to be used. Flotemersch and Blocksom (2005) further investigated the appropriate sample length (1000 m versus 2000 m on single or paired banks) for electrofishing methods in large river bioassessments. At each bank at each of 60 sites in the Ohio River basin, USA, a distance of 40 times the wetted channel width (up to 2000 m) was electrofished (in an upstream to downstream direction) at each of 10 equally spaced transects. Based on fish richness metrics, they found that electrofishing 1000 m of shoreline (along one bank or shared along paired banks) was sufficient to capture the assemblage. In very deep rivers (> 4 m) however, they recommended that night-time sampling or increased day-time sampling distances may be required. For large rivers that have many major dams or lock structures along their length, sample distance and effort for fish bioassessments may be adapted to sampling within reservoirs or navigational pools, as shown by Blocksom et al. (2009). This study examined the results of night-time electrofishing conducted along 500 m lengths of multiple sites within each navigational pool (created by a dam or lock) on the Ohio River, USA, to determine the number of sites and samples needed to collect ~90% of the total fish 30 species observed within a pool, and to minimize variation in metrics calculated from the fish species counts. They found that sampling from 15 sites satisfied both criteria. Thus, the number of sites needed to be sampled along a large river’s length to effectively assess taxonomic richness may also be important to establish for assessments based on biotic metrics. As Hughes et al. (2011) state, an insufficient number of sites will underestimate taxonomic richness (and may therefore confound health assessment) but too many sites will incur unnecessary cost and effort. Thus, they devised a study to determine the number of sites and sampling effort needed to collect 90– 95% of the fish, amphibian, macroinvertebrate and diatom taxa collected from intensive surveys of nonwadeable rivers in the USA, 75–95% of the time. Results were based on surveys from a random selection of 20 sites on each river’s main stem and indicated that, for the studied rivers, 12–16 randomly distributed sites would provide cost-efficient estimates of fish and amphibian richness, but that 20 sites underestimated macroinvertebrate and diatom richness. For any one large river, it may therefore be necessary to conduct trial field studies, e.g. by following similar methods to those of Hughes et al. (2011), to establish the appropriate number of sites needed for a river health assessment program that uses (or may use) metrics based on taxonomic richness. Flotemersch et al. (2011) reviewed the literature on appropriate site length for bioassessments of large rivers (determined by biological or physical criteria), including many of the studies discussed above, and concluded that the objectives, scope and standards of the river health assessment program ultimately determine the site length sufficient for biologically-based surveys, along with the particular habitat heterogeneity of the river/s of interest. Where possible, pilot studies should therefore be conducted to determine the sampling effort required to adequately capture the biological characteristics of a river. Flotemersch et al. (2006b) also discussed options for and implications of study design in large river bioassessments, including pros and cons of biological- versus physical-based approaches to determining reach length. They suggested that the scale of the assessment (e.g. a site-specific assessment versus an assessment of all non-wadeable sections of a river) should also determine sampling effort and study design. Large lakes and reservoirs Eutrophication of lakes and reservoirs due to anthropogenic disturbance of river systems and land is worldwide phenomenon (Sondergarrd and Jeppesen 2007). As such, many of the sampling approaches to detecting ecological responses to human disturbance in lakes and reservoirs have focused on measures of surface and bottom water quality (see below). There appears not to have been major or explicit documentation of, or discussion on, site selection and sampling effort for lake and reservoir health assessment in the readily accessible, English-language scientific literature. However, the ecological studies of lake, reservoir and river systems that are reviewed below, in terms of field and laboratory methods used in the assessment of biotic parameters, may also provide insight into study design and sampling effort options for bioassessment of large water bodies, including that of large lakes and reservoirs. 31 6. Selection of appropriate and responsive indicators To be included in a river health assessment, indicators need to satisfy several criteria, regardless of whether the assessment is for small streams or large water bodies (e.g. Rapport et al. 1998). They should be relevant to the specific questions asked by the assessment program, strongly associated and/or responsive to the putative impact/s, ecologically and/or socially important, and efficient to measure (Downes et al. 2002). The approach to selecting indicators and the thresholds and target values are outlined in the Guiding Principles above. Accordingly, it is not appropriate to be prescriptive here as to which indicators are most appropriate. However, given the broad range of stressors impacting rivers and lakes in China and the growing interest in improving ecological health in these systems, the following general comments can be made. 6.1. Abiotic indicators Of the abiotic indicators considered here: hydrology, water quality, and physical form, the first two groups are obvious candidates for inclusion in river health assessments programs for large rivers and lakes in China. Not only do they underpin relevant aspects of ecological health, but they are also can be used to directly guide appropriate management actions. Another major advantage is that both are usually already measured through established programs and the field and laboratory methods are well documented. All that is required is selection of appropriate indicators and analysis of data. Physical form can be more difficult to measure and interpret than hydrology and water quality. However, physical form has close links to ecological health, and it is an aspect of rivers that attracts significant management investment (through bank protection works, levee/dyke construction, grade control structures, and sediment mining). Given the difficulty of measuring physical form in the field using objective measurement techniques (Gippel et al. 2011b), we would recommend the use of remote sensing technologies, and documentation of direct actions on rivers that affect physical form (such as levee/dyke construction, erosion control, and sediment mining). Rivers are generally considered to be open ecosystems, highly influenced by their surroundings and by abiotic disturbance, particularly hydrologic regime. In contrast, lakes are more stable, more isolated and more biologically controlled (Dent et al. 2002). This suggests that, in general, the inclusion of abiotic indicators in a health assessment program is less important for lakes than for large rivers. However, for any particular lake, the value of abiotic indicators could depend on site-specific issues. In addition, as eutrophication is a major, anthropogenically-induced, problem for lakes and reservoirs throughout the world (Sondergaard and Jeppessen 2007), ecological health assessment that incorporates the use of water quality indicators may be particularly important. 6.2. Biotic indicators In the context of large rivers, indicators of ecological health need to reflect relationships or responses to human disturbance as distinct from effects of river size (sensu Wilhelm et al. 2005; e.g. Schletterer et al. 2010). For example, a fish species adapted to and prevalent in small headwater reaches is unlikely to be found in great numbers in the lowland, non-wadeable reaches of a river regardless of human disturbance factors. Likewise, indicators based on ecosystem metabolism may need to distinguish between natural and anthropogenically-induced turbidity in large water bodies, as well as the effect of channel width on water-column shading by riparian vegetation. The use of biological traits (e.g. sensitivity to pesticides, Schletterer et al. 2010) and measures of body condition or external deformities (e.g. in fish, Stevenson and Woods 2006; Blocksom et al. 2009) as stressor-specific response indicators may also help to isolate ecological responses to anthropogenic stress from responses to natural environmental factors. 32 Another factor to consider, in the context of large river health assessment and indicators based on biotic assemblages, is that of relative abundance or diversity. In large rivers heavily affected by human disturbances, inclusion of dominant taxa in biotic measures (e.g. in those based on macroinvertebrates) may mask ecological responses to stressors and/or management actions, especially the responses of the more sensitive taxa (Jackson et al. 2010). For macroinvertebrates, the effect of dominance on health assessment is mediated through the combined effects of habitat preferences and pollution tolerance, i.e. biological traits. For example, assemblages in certain large (high-order) rivers may be dominated by a few taxa that are suited to habitats with naturally high amounts of suspended or benthic fine particulate organic matter, such as oligochaetes and filter feeders (Jackson et al. 2010). However, these taxa are also usually pollution tolerant and bioassessment metrics based on these taxa and/or their pollution tolerance values may potentially lead to erroneous conclusions about river health. Jackson et al. (2010) investigated the effect of including or excluding dominant taxa on macroinvertebrate-based metrics commonly used in large river bioassessments in the USA. They found that removing dominant taxa, which, in their study, included oligochaetes and hydropsychid caddisflies, improved the accuracy of many metrics and the interpretability in terms of assessing river impairment. The authors also suggested that removal of other taxa, such as chironomid midges and invasive species, which may dominate the assemblage of any one large river, could also improve metric performance but that a suitable definition must be first established of when dominance is large enough to hinder the accuracy of that river’s health assessment. Furthermore, and at the opposite end of the dominance continuum, it may be important to include, rather than exclude, ‘rare’ taxa in bioassessments of large rivers, as this has been considered an important factor in wadeable stream bioassessment (see Blocksom and Johnston 2009; Weigel and Dimick 2011). However, greater sampling effort may be required to capture rare species within large water bodies compared with smaller ones (Dolph et al. 2010). Conversely, in the assessment of lakes and reservoirs, the inclusion of indicators based on dominant biota, which include many toxic cyanobacteria species, may be of particular importance and relevance in ecological health assessments as phytoplankton blooms are often a key indication of an anthropogenically-disturbed lake ecosystem (Schindler 2000). 33 7. Conclusions This review of literature revealed a number of key points that require consideration when designing a program for assessment of the ecological health of large freshwater water bodies (rivers, lakes and reservoirs): • Most large water bodies are significantly impaired by anthropogenic disturbance, so best attainable condition may be the most appropriate reference. • A combination of biotic and abiotic indicators are needed in assessment of the health of large water bodies. • It is important to define and measure human disturbances on spatial and temporal scales relevant to the ecosystem and/or ecosystem health indicator of interest. • Attributes of large water bodies that are relevant to ecological health can be measured using field sampling and increasingly using remote sensing technologies. • Field sampling of large water bodies, compared with smaller wadeable streams, could involve considerably more effort per site (due to the larger area that needs to be covered), but, ultimately this will depend on project objectives, scope and standards, plus the physical heterogeneity of the water body in question. • Where possible, and relevant, pilot studies should be conducted to determine the sampling effort required to adequately capture the biological characteristics of the water body in question. • The principles of monitoring hydrology for river health assessment are the same regardless of the size of the river, but in large rivers affected by weirs, it may also be important to measure water level variability (in addition to discharge variability). • For lakes and reservoirs, the pattern of water level is the key hydrological concern. However, in some systems it may also be necessary to monitor circulation, as affected by wind, inflows, and thermal differences. • The physical form of large rivers is difficult to measure objectively, with the visual estimation approach being particularly unreliable. The alternative is to measure surrogates (aspects of hydrology that are linked to channel stability), or to use remote sensing technologies. • Water quality parameters are important indicators in ecological health assessment of large water bodies. Although regular field sampling is necessary for some aspects of water quality, much can be achieved through the use of continuously recording probes, and remote sensing technologies. • Useful biotic indicator groups for large water bodies are diatoms and algae, macroinvertebrates, fish, and vegetation. Measures of ecosystem metabolism and other functional indicators (e.g. fish body condition) are also of relevance. While diatoms and algae, macroinvertebrates and fish require field sampling, remote sensing methodologies are wellestablished for measuring attributes of vegetation. • In the context of large rivers, indicators of ecological health need to reflect relationships or responses to human disturbance as distinct from effects of river size. • In large rivers heavily impaired by human disturbances, inclusion of dominant taxa in biotic measures (and indicators based on macroinvertebrates in soft-sediment systems) may mask ecological responses to stressors and/or management actions, especially the responses of other indicators and the more sensitive taxa. 34 • In the assessment of lakes and reservoirs, the inclusion of indicators based on dominant biota, e.g. cyanobacteria, may be of particular importance and relevance in ecological health assessments as phytoplankton blooms are often a key indication of an anthropogenicallydisturbed lake ecosystem. This review raised a number of technical issues concerning assessment of the ecological health of large water bodies. These issues might mean that different approaches are needed for large water bodies compared with wadeable streams. There are other practical issues not covered in this review that could influence design of the sampling program for large water bodies. Most of the practical issues relate to the need for boats in field sampling, which can involve: additional staff, access and launching requirements, management of occupational health and safety risks, training and licensing of boat crews, and longer times required per site. Studies have demonstrated that remote sensing may be more efficient and cost-effective than field sampling, but some indicators cannot be measured by remote sensing. In designing an ecological health assessment program for large water bodies it is necessary then to trade off the expediency of remote sensing against the detail that is possible with field-based sampling. Finally, the choice of methods will ultimately depend on the objectives of the program, the funding available, and the skills of the staff. These considerations could lead to adoption of a program that includes both field-based and remote sensing approaches, plus a research component that seeks to develop the most effective technologies and is used to continually improve the program. 35 8. References Angradi T. R., Pearson, M. S., Jicha, T. M., Taylor, D. L., Bolgrien, D. W., Moffett, M. F., Blocksom, K. A. and Hill, B. H.. 2009. Using stressor gradients to determine reference expectations for great river fish assemblages. Ecological Indicators 9: 748-764. Ashraf, S., Brabyn, L. and Hicks, B. J. 2007. Remote sensing of freshwater habitats for large rivers and lakes of the Waikato region using sub-pixel classification. CBER Contract Report No. 63. Client report prepared for Environment Waikato. Centre for Biodiversity and Ecology Research, Department of Biological Sciences, School of Science and Engineering, The University of Waikato, Hamilton, New Zealand. URL: http://cber.bio.waikato.ac.nz/PDFs/CBER_63.pdf (accessed 19/08/2011). Baban, S. M. J. 1993. Detecting water quality parameters in the Norfolk Broads, U.K., using Landsat imagery. International Journal of Remote Sensing 14: 1247-1267. Bady, P., Doledec, S., Fesl, C., Gayraud, S., Bacchi, M. and Scholl, F. 2005. Use of invertebrate traits for the biomonitoring of European large rivers: the effects of sampling effort on genus richness and functional diversity. Freshwater Biology 50: 159-173. Baker, J. R., Peck, D. V. and Sutton, D. W. (editors). 1997. Environmental Monitoring and Assessment Program–Surface Waters: Field operations manual for lakes. EPA/620/R-97/001. U.S. Environmental Protection Agency, Corvallis, Oregon, USA. Barbour, M. T., Gerritsen, J., Snyder, B. D. and Stribling, J. B. 1999. Rapid bioassessment protocols for use in streams and wadeable rivers: periphyton, benthic macroinvertebrates and fish. Second edition. EPA 841-B-99-002. U.S. Environmental Protection Agency, Office of Water, Washington, DC, USA. Battle, J. M., Jackson, J. K. and Sweeney, B. W. 2007. Mesh size affects macroinvertebrate descriptions in large rivers: examples from the Savannah and Mississippi Rivers. Hydrobiologia 592: 329-343. Baxter, C. V., Fausch, K. D. and Saunders, W. C. 2005. Tangled webs: reciprocal flows of invertebrate prey link streams and riparian zones. Freshwater Biology 50: 201-220. Biedenharn, D. S. and Watson, C. C. 1997. Stage adjustment in the lower Mississippi River, USA. Regulated Rivers: Research and Management 13: 517-536. Blench, T. 1969. Mobile-Bed Fluviology. The University of Alberta Press, Edmonton, Alberta, Canada. Blocksom, K. A. and Johnson, B. R. 2009. Development of a regional macroinvertebrate index for large river bioassessment. Ecological Indicators 9: 313-328. Blocksom, K. and Flotemersch, J. 2005. Comparison of macroinvertebrate sampling methods for nonwadeable streams. Environmental Monitoring and Assessment 102: 243-262. Blocksom, K., Emery, E. and Thomas, J. 2009. Sampling effort needed to estimate condition and species richness in the Ohio river, USA. Environmental Monitoring and Assessment 155: 157167. Blukacz, E. A., Shuter, B. J. and Sprules, W. G. 2009. Towards understanding the relationship between wind conditions and plankton patchiness. Limnology and Oceanography 54: 1530–1540 Bond, N., Wei, L., Shichuang, W., Speed, R., Gippel, C., Catford, J., Bunn, S. and Close, P. 2011. ACEDP Sub-Project Report: Assessment of River Health in the Pearl River Basin (Gui SubCatchment). International WaterCentre and the Chinese Research Academy of Environmental Science, Brisbane, Australia. 36 Booth, D. T., Cox, S. E. and Simonds, G. 2007. Riparian monitoring using 2-cm GSD aerial photography. Ecological Indicators 7: 636-648. Boulton, A. J. 1999. An overview of river health assessment: philosophies, practice, problems and prognosis. Freshwater Biology 41: 469-479. Bowen, Z. H. and Waltermire, R. G. 2002. Evaluation of light detection and ranging (LIDAR) for measuring river corridor topography. Journal of the American Water Resources Association 38: 33-41. Bunn, S. E. and Arthington, A. H. 2002. Basic principles and ecological consequences of altered flow regimes for aquatic biodiversity. Environmental Management 30: 492-507. Bunn, S. E., Davies, P. M. and Mosisch, T. D. 1999. Ecosystem measures of river health and their response to riparian and catchment degradation. Freshwater Biology 41: 333-345. Chételat, J., F. R. Pick, and P. B. Hamilton. 2006. Potamoplankton size structure and taxonomic composition: influence of river size and nutrient concentrations. Limnology and Oceanography 51: 681-689. Congalton, R. G., Birch, K., Jones, R. and Schreiver, J. 2002. Evaluating remotely sensed techniques for mapping riparian vegetation. Computers and Electronics in Agriculture 31: 113-126. Crome, F. H. J. 1988. To drain or not to drain? - Intermittent swamp drainage and waterbird breeding. Emu 88: 243-248. De Ceballos, B. Konig, S. O., A. and De Oliveira, J. F. 1998. Dam reservoir eutrophication: a simplified technique for a fast diagnosis of environmental degradation. Water Research 32: 3477-3483. DeGagnea, M. P. J., Douglas, G. G., Hudson, H. R. and Simonovic, S. P. 1996. A decision support system for the analysis and use of stage-discharge rating curves. Journal of Hydrology 184: 225-241. Dent, C. L., Cumming, G. S. and Carpenter, S. R. 2002. Multiple states in river and lake ecosystems. Philosophical Transactions of the Royal Society B: Biological Sciences 357: 635–645. Desgranges, J-L, Ingram, J., Droleri, B., Morin, J. Savage, C and Borcard, D. 2006. Modelling wetland bird response to water level changes in the Lake Ontario-St Lawrence River Hydrosystem. Environmental Monitoring and Assessment 113: 329–365. Dixit, S. S., Smol, J. P., Charles, D. F., Hughes, R. M., Paulsen, S. G. and Collins, G. B. 1999. Assessing water quality changes in the lakes of the northeastern United States using sediment diatoms. Canadian Journal of Fisheries and Aquatic Sciences 56: 131-152. Downes, B. J. 2010. Back to the future: little-used tools and principles of scientific inference can help disentangle effects of multiple stressors on freshwater ecosystems. Freshwater Biology 55: 6079. Downes, B. J., Barmuta, L. A., Fairweather, P. G., Faith, D. P., Keough, M. J., Lake, P. S., Mapstone, B. D. and Quinn, G. P. 2002. Monitoring Ecological Impacts: Concepts and Practice in Flowing Waters. Cambridge University Press, Cambridge, U.K. Fellows, C., Wos, M., Pollard, P. and Bunn, S. 2007. Ecosystem metabolism in a dryland river waterhole. Marine and Freshwater Research 58: 250-262. Flotemersch, J. E., Stribling J. B., and Paul, M. J. 2006b. Concepts and approaches for the bioassessment of non-wadeable streams and rivers. U.S. Environmental Protection Agency, Washington, DC, USA. Flotemersch, J. E., Stribling, J. B., Hughes, R. M., Reynolds, L., Paul, M. J. and Wolter, C. 2011. Site length for biological assessment of boatable rivers. River Research and Applications 27: 520535. 37 Flotemersch, J. E., Blocksom, K., Hutchens, J. J. and Autrey, B. C. 2006a. Development of a standardized large river bioassessment protocol (LR-BP) for macroinvertebrate assemblages. River Research and Applications 22: 775-790. Flotemersch, J., and Blocksom, K. 2005. Electrofishing in boatable rivers: does sampling design affect bioassessment metrics? Environmental Monitoring and Assessment 102: 263-283. Fore, L. S. and Grafe, C. 2002. Using diatoms to assess the biological condition of large rivers in Idaho (USA). Freshwater Biology 47: 2015-2037. Gippel, C.J. 2010. River Health Monitoring, Assessment and Applications. Technical Report 1. ACEDP – River Health and Environmental Flow in China. ACEDP Activity No: P0018. International Water Centre, Brisbane, Australia. Gippel, C.J., Zhang, Y., Qu, X., Kong, W., Bond, N. R., Jiang, X. and Liu, W. 2011a. River health assessment in China: comparison and development of indicators of hydrological health. ACEDP Technical Report 4. Australia-China Environment Development Partnership, River Health and Environmental Flow in China. The Chinese Research Academy of Environmental Sciences, the Pearl River Water Resources Commission and the International WaterCentre, Brisbane, Australia. Gippel, C.J., Catford, J., Bond, N. R., Zhang, Y., Qu, X., Kong, W., and Liu, W. 2011b. River health assessment in China: development of physical form indicators. ACEDP Technical Report 5. Australia-China Environment Development Partnership, River Health and Environmental Flow in China. The Chinese Research Academy of Environmental Sciences, the Pearl River Water Resources Commission and the International WaterCentre, Brisbane, June. Goetz, S. J. 2006. Remote sensing of riparian buffers: past and future prospects. Journal of the American Water Resources Association 42: 133-143. Hamilton, S.K, Kellndorfer, J., Lehner, B. and Tobler, M. 2007. Remote sensing of floodplain geomorphology as a surrogate for biodiversity in a tropical river system (Madre de Dios, Peru). Geomorphology 89: 23-38. Herlihy, A. T. and Hendricks, C. W. 2000. Water chemistry and microbiology. In J. M. Lazorchak, B. H. Hill, D. K. Averill, D. V. Peck, and D. J. Klemm (editors). Environmental Monitoring and Assessment Program—Surface Waters: Field Operations and Methods for Measuring the Ecological Condition of Non-Wadeable Rivers and Streams. U.S. Environmental Protection Agency, Cincinnati, Ohio, USA. Hill, B. H. and Herlihy, A. T. 2000. Periphyton. In J. M. Lazorchak, B. H. Hill, D. K. Averill, D. V. Peck, and D. J. Klemm (editors). Environmental Monitoring and Assessment Program—Surface Waters: Field Operations and Methods for Measuring the Ecological Condition of NonWadeable Rivers and Streams. U.S. Environmental Protection Agency, Cincinnati, Ohio, USA. Hilldale, R. C. and Raff, D. 2008. Assessing the ability of airborne LiDAR to map river bathymetry. Earth Surface Process and Landforms 783: 773-783. Hughes, R. M., Herlihy, A. T., Gerth, W. J. and Pan. Y. 2011. Estimating vertebrate, benthic macroinvertebrate, and diatom taxa richness in raftable Pacific Northwest rivers for bioassessment purposes. Environmental Monitoring and Assessment, doi: 10.1007/s10661011-2181-9 Hunsaker, C. T. and Levine, D. A. 1995. Hierarchical approaches to the study of water quality in rivers. Bioscience 45: 193-203. Izagirre, O., Agirre, U. Bermejo, M. Pozo, J. and Elosegi, A. 2008. Environmental controls of wholestream metabolism identified from continuous monitoring of Basque streams. Journal of the North American Benthological Society 27: 252–268. 38 Jackson, J. K., Battle, J. M. and Sweeney, B. W. 2010. Monitoring the health of large rivers with macroinvertebrates: do dominant taxa help or hinder the assessment? River Research and Applications 26: 931-947. Johansen, K., Phinn, S., Dixon, I., Douglas, M. and Lowry, J. 2007. Comparison of image and rapid field assessments of riparian zone condition in Australian tropical savannas. Forest Ecology and Management 240: 42-60. Johnson, B. L., Richardson, W. B. and Naimo, T. J. 1995. Past, present, and future concepts in large river ecology. Bioscience 45:134. Julian, J.P. and Torres, R. 2006. Hydraulic erosion of cohesive riverbanks. Geomorphology 76: 193– 206. Jung, H. C., Hamski, J., Durand, M., Alsdorf, D., Hossain, F., Lee, H., Hossain, A. K. M. A., Hasan, K., Khan, A. S., and Hoque, A. K. M. Z. 2010. Characterization of complex fluvial systems using remote sensing of spatial and temporal water level variations in the Amazon, Congo, and Brahmaputra Rivers. Earth Surface Processes and Landforms 35: 294-304. Kellerhals, R., and Church, M. 1989. The morphology of large rivers: characterization and management. In D. P. Dodge (editor). Proc. of the International Large River Symposium. Canadian Special Publication of Fisheries and Aquatic Science 106: 31-48. Kelly, M. G. and Whitton, B. A. 1995. The trophic diatom index: a new index for monitoring eutrophication in rivers. Journal of Applied Phycology 7: 433-444. Kloiber, S. N., Brezonik, P. L. Olmanson, L. G. and Bauer, M. E. 2002. A procedure for regional lake water clarity assessment using Landsat multispectral data. Remote Sensing of Environment 82: 38-47. Knoll, L. B., Vanni, M. J. and Renwick, W. H. 2003. Phytoplankton primary production and photosynthetic parameters in reservoirs along a gradient of watershed land use. Limnology and Oceanography 48: 608–617. Lammert, M. and Allan, J. D. 1999. Assessing biotic integrity of streams: effects of scale in measuring the influence of land use/cover and habitat structure on fish and macroinvertebrates. Environmental Management 23: 257-270. Lane, C. R., Flotemersch, J. E., Blocksom, K. A. and Decelles, S. 2007. Effect of sampling method on diatom composition for use in monitoring and assessing large river condition. River Research and Applications 23: 1126-1146. Lazorchak, J. M., Hill, B. H., Averill, D. K., Peck, D. V. and Klemm, D. J. (editors). 2000. Environmental Monitoring and Assessment Program-Surface Waters: Field Operations and Methods for Measuring the Ecological Condition of Non-Wadeable Rivers and Streams. U.S. Environmental Protection Agency, Cincinnati, Ohio, USA. Leckie, D. G., Cloney, D., Jay, C and Paradine, D. 2005. Automated mapping of stream features with high-resolution multispectral imagery: an example of the capabilities. Photogrammetric Engineering & Remote Sensing 71: 145–155. Leigh, C., Burford, M. A., Roberts, D. T. and Udy, J. W. 2010b. Predicting the vulnerability of reservoirs to poor water quality and cyanobacterial blooms. Water Research 44: 4487-4496. Leigh, C., Sheldon, F., Kingsford, R. T. and Arthington, A. H. 2010a. Sequential floods drive 'booms' and wetland persistence in dryland rivers: a synthesis. Marine and Freshwater Research 61: 896908. Leigh, C., Qu, X., Zhang, Y., Kong, W., Meng, W., Hanington, P., Speed, R., Gippel, C., Bond, N., Catford, J., Bunn, S. and Close, P.. 2011. ACEDP Project Report: Assessment of River Health 39 in the Liao River Basin (Taizi Sub-Catchment). International WaterCentre and the Chinese Research Academy of Environmental Science, Brisbane, Australia. Li, H. W. and Li, J. L. 1996. Fish community composition. In F. R. Hauer and G. A. Lamberti (editors). Methods in Stream Ecology. Academic Press, San Diego, USA. Liu, X. and Wang, H. 2010. Estimation of minimum area requirement of river-connected lakes for fish diversity conservation in the Yangtze River floodplain. Diversity and Distributions 16: 932-940. Luck, M., Maumenee, N., Whited, D., Lucotch, J., Chilccote, S., Lorang, M., Goodman, D., McDonald, K., Kimball, J. and Standford, J. 2010. Remote sensing analysis of physical complexity of North Pacific Rim rivers to assist wild salmon conservation. Earth Surface Processes and Landforms 35: 1330–1343. Maheu-Giroux, M. and Blois, S. D. 2005. Mapping the invasive species Phragmites australis in linear wetland corridors. Aquatic Botany 83: 310-320. Malthus, T. J. and George, D. G. 1997. Airborne remote sensing of macrophytes in Cefni Reservoir, Anglesey, UK. Aquatic Botany 58: 317-332. Malthus, T. J. and Karpouzli, E. 2003. Integrating field and high spatial resolution satellite-based methods for monitoring shallow submersed aquatic habitats in the Sound of Eriskay, Scotland, UK. International Journal of Remote Sensing 24: 2585-2593. Mancini, K. M. and Rusch, O. H. 1988. Indices to distribution and abundance of some inconspicuous waterbirds at Horicon Marsh. Journal of Field Ornithology 59: 67-75. Martinez, J. M., Guyot, J. L., Cochonneau, G. and Seyler, F. 2007. Surface water quality monitoring in large rivers with MODIS data application to the Amazon basin. Geoscience and Remote Sensing Symposium, 2007. IGARSS 2007. IEEE International, pp. 4566 – 4569. Meador, M. R. 2005. Single-pass versus two-pass boat electrofishing for characterizing river fish assemblages: Species richness estimates and sampling distance. Transactions of the American Fisheries Society 134: 59-67. Miller, S. N., Shrestha, S. R. and Semmens, D. 2004. Semi-automated Extraction and Validation of Channel Morphology from LIDAR and IFSAR Terrain Data. In Proceedings of the ASPRS Annual Conference, Denver, Colorado, USA. URL: sites.google.com/site/sudhirraj/ASPRSPaper.pdf (accessed 18/08/2011). Minns, C. K., Cairns, V. W., Randall, R. G. and Moore, J. E. 1994. An Index of Biotic Integrity (IBI) for fish assemblages in the littoral-zone of Great-Lakes areas of concern. Canadian Journal of Fisheries and Aquatic Sciences 51: 1804-1822. Moiseenko, T. I., Voinov, A. A., Megorsky, V. V., Gashkina, N. A., Kudriavtseva, L. P., Vandish, O. I., Sharov, A. N., Sharova, Y. and Koroleva, I. N. 2006. Ecosystem and human health assessment to define environmental management strategies: The case of long-term human impacts on an Arctic lake. Science of the Total Environment 369:1-20. Muller, E. 1997. Mapping riparian vegetation along rivers: old concepts and new methods. Aquatic Botany 58: 411-437. Narulamani, S., Zhou, Y. C. and Jensen, J. R. 1997. Application of remote sensing and geographic information systems to the delineation and analysis of riparian buffer zones. Aquatic Botany 58: 393-409. Nechad, B., Ruddick, K. G., and Park, Y. 2010, Calibration and validation of a generic multisensor algorithm for mapping of total suspended matter in turbid waters: Remote Sensing of Environment 114: 854-866. 40 Neebling, T. E. and Quist, M. C. 2011. Comparison of boat electrofishing, trawling, and seining for sampling fish assemblages in Iowa's nonwadeable rivers. North American Journal of Fisheries Management 31: 390-402. Nelson, S. A. C., Cheruvelil, K. S. and Soranto, P. A. 2006. Satellite remote sensing of freshwater macrophytes and the influence of water clarity. Aquatic Botany 85: 289-298. Northwest Hydraulic Consultants Ltd 2003. Nechako River geomorphic assessment phase I: historical analysis of Lower Nechako River, Final Report. BC Ministry of Water, Land and Air Protection, Victoria, British Colombia, Canada. O'Connor, R. J., Walls, T. E. and Hughes, R. M. 2000. Using multiple taxonomic groups to index the ecological condition of lakes. Environmental Monitoring and Assessment 61: 207-228. Oestlund, C., Flink, P., Stroembeck, N., Pierson, D. and Lindell, T. 2001. Mapping of the water quality of Lake Erken, Sweden, from Imaging Spectrometry and Landsat Thematic Mapper. The Science of the Total Environment 268: 139-154. Parsons, M. and Norris, R. 1996. The effect of habitat-specific sampling on biological assessment of water quality using a predictive model. Freshwater Biology 36: 419-434. Peterson, E. E., Sheldon, F., Darnell, R., Bunn, S. E. and Harch, B. D. 2011. A comparison of spatially explicit landscape representation methods and their relationship to stream condition. Freshwater Biology 56: 590-610. Pinter, N. and Heine, R. 2005. Hydrodynamic and morphodynamic response to river engineering documented by fixed-discharge analysis, Lower Missouri River, USA. Journal of Hydrology 302: 70–91. Poff, N. L., Allan, J. D., Bain, M. B., Karr, J. R., Prestegaard, K. L., Richter, B. D., Sparks, R. E. and Stromberg, J. C. 1997. The natural flow regime: a paradigm for river conservation and restoration. Bioscience 47: 769-784. Porter, S. D, Cuffney, T. F, Gurtz, M. E and Meador, M. R. 1993. Methods for collecting algal samples as part of the National Water-Quality Assessment Program. Report 93-409. U.S. Geological Survey, Raleigh, North Carolina; USA. Raitala, J., Janunen, H. and Lampinen, J. 1985. Application of landsat satellite data for mapping aquatic areas in north-eastern Finland. Aquatic Botany 21: 285-294. Rapport, D. J., Costanza, R. and McMichael, A. J. 1998. Assessing ecosystem health. Trends in Ecology and Evolution 13: 397-402. Reavie, E. D., Jicha, T. M., Angradi, T. R., Bolgrien, D. W. and Hill, B. H. 2010. Algal assemblages for large river monitoring: comparison among biovolume, absolute and relative abundance metrics. Ecological Indicators 10: 167-177. Rehm, E. M. and Baldassarre, G. A. 2007. The influence of interspersion on marsh bird abundance in New York. The Wilson Journal of Ornithology 119: 650-656. Roth, N. E, Southerland, M. T., Chaillou, J. C., Kazyak, P. F. and Stranko, S. A. 2000. Refinement and validation of a fish index of biotic integrity for Maryland streams. Versar, Inc. Columbia and Maryland Department of Natural Resources, Annapolis, Maryland, USA. URL: www.dnr.state.md.us/streams/pdfs/ea-00-2_fibi.pdf (accessed 18 August 2011). Scott, A. 1997. Relationship between waterbird ecology and river flows in The Murray-Darling Basin. CSIRO Technical report 5/97, Commonwealth Scientific and Industrial Research Organisation, Canberra, Australia. 41 Schletterer, M., Fureder, L., Kuzovlev, V. V. and Beketov, M. A. 2010. Testing the coherence of several macroinvertebrate indices and environmental factors in a large lowland river system (Volga River, Russia). Ecological Indicators 10: 1083-1092. Senay, G. B., Shafique, N. A., Autrey, B. C., Fulk, F. and Cormier, S. M. 2001. The selection of narrow wavebands for optimizing water quality monitoring on the Great Miami River, Ohio using hyperspectral remote sensor data. Journal of Spatial Hydrology 1: 1-22. Shafique, N. A., Fulk, F., Autrey, B. C. and Flotemersch, J. 2003. Hyperspectral remote sensing of water quality parameters for large rivers in the Ohio River Basin. In Renard, K. G., McElroy, S. A., Gburek, W. J., Canfield, H. E. and Scott, R. L. (editors), Proceedings of the First Interagency Conference on Research in the Watersheds, pp. 216-221. USDA Agricultural Research Service, Washington, DC, USA. URL: http://www.tucson.ars.ag.gov/icrw/proceedings/shafique.pdf (accessed 18 August 2011). Shanmugam, P., Ahn, Y.-H. and Sanjeevi, S. 2006. A comparison of the classification of wetland characteristics by linear spectral mixture modelling and traditional hard classifiers on multispectral remotely sensed imagery in southern India. Ecological Modelling 194: 379-394. Smith, A. J. and Tran, C. P. 2011. A weight-of-evidence approach to define nutrient criteria protective of aquatic life in large rivers. Journal of the North American Benthological Society 29: 875-891. Sondergaard, M., and Jeppesen, E. 2007. Anthropogenic impacts on lake and stream ecosystems, and approaches to restoration. Journal of Applied Ecology 44: 1089-1094. Stevenson, R. D. and Woods, W. A. 2006. Condition indices for conservation: new uses for evolving tools. Integrative and Comparative Biology 46: 1169-1190. Stoddard, J. L., Larsen, D. P., Hawkins, C. P., Johnson, R. K. and Norris, R. H. 2006. Setting expectations for the ecological condition of streams: the concept of reference condition. Ecological Applications 16: 1267-1276. Tassan, S. 1997. A numerical model for the detection of sediment concentration in stratified river plumes using Thematic Mapper data. International Journal of Remote Sensing 18: 2699-2705. Tyler, A.N., Svab, E., Preston, T., Presing, M. and Kovacs, W. A. 2006. Remote sensing of the water quality of shallow lakes: a mixture modelling approach to quantifying phytoplankton in water characterized by high-suspended sediment. International Journal of Remote Sensing 27: 15211537. Utrup, N. J., and Fisher, W. L. 2006. Development of a rapid bioassessment protocol for sampling fish in large prairie rivers. North American Journal of Fisheries Management 26: 714-726. Valley, R. D., Drake, M. T. and Anderson, C. S. 2005. Evaluation of alternative interpolation techniques for the mapping of remotely-sensed submersed vegetation abundance. Aquatic Botany 81: 1325. Valta-Hulkkonen, K., Pellikka, P., Tanskenen, H., Ustinov, A. and Sandman, O. 2003. Digital false colour aerial photographs for discrimination of aquatic macrophyte species. Aquatic Botany 75: 71-88. Vannote, R. L., Minshall, G. W., Cummins, K. W., Sedell, J. R. and Cushing, C. E. 1980. The river continuum concept. Canadian Journal of Fisheries and Aquatic Sciences 37: 130-137. Wang, J. J., Lu, X. X., Liew, S. C. and Zhou, Y. 2009. Retrieval of suspended sediment concentrations in large turbid rivers using Landsat ETM plus: an example from the Yangtze River, China: Earth Surface Processes and Landforms 34: 1082-1092. Wang, J. J., Lu, X. X., Liew, S. C. and Zhou, Y. 2010. Remote sensing of suspended sediment concentrations of large rivers using multi-temporal MODIS images: an example in the Middle and Lower Yangtze River, China. International Journal of Remote Sensing 31: 1103-1111. 42 Weberm R. M. and Dunno, G. A. 2001. Riparian vegetation mapping and image processing techniques, Hopi Indian Reservation, Arizona. Photogrammetric Engineering and Remote Sensing 67: 179186. Weigel, B. M., and D. M. Robertson. 2007. Identifying biotic integrity and water chemistry relations in nonwadeable rivers of Wisconsin: toward the development of nutrient criteria. Environmental Management 40: 691-708. Weigel, B. M., and Dimick, J. J. 2011. Development, validation, and application of a macroinvertebratebased Index of Biotic Integrity for nonwadeable rivers of Wisconsin. Journal of the North American Benthological Society 30: 665-679. Weigel, B. M., Lyons, J., Rasmussen, P. W. and Wang, L. Z. 2006. Relative influence of environmental variables at multiple spatial scales on fishes in Wisconsin's warmwater nonwadeable rivers. In R. M. Hughes, L. Wang and P. W. Seelbach (editors). Landscape Influences on Stream Habitats and Biological Assemblages. American Fisheries Society, Bethesda, Maryland, USA. Wessell, K. J., Merritt, R. W., Wilhelm, J. G. O., Allan, J. D., Cummins, K. W. and Uzarski, D. G. 2008. Biological evaluation of Michigan's non-wadeable rivers using macroinvertebrates. Aquatic Ecosystem Health and Management 11: 335-351. Whited, D., Stanford, J. A. and Kimball, J. S. 2002. Application of airborne multispectral digital imagery to quantify riverine habitats at different baseflows. River Research and Applications 18: 583594. Whitton, B.A., Rott, E. and Friedrich, G. 1991. Use of Algae for Monitoring Rivers. Institut für Botanik, Universität Innsbruck, Innsbruck, Austria. Wilhelm, J. G. O., Allan, J. D., Wessell, K. J., Merritt, R. W. and K. W. Cummins. 2005. Habitat assessment of non-wadeable rivers in Michigan. Environmental Management 36: 592-609. Wright, A., Marcus, W. A. and Aspinall, R. 2000. Evaluation of multispectral, fine scale digital imagery as a tool for mapping stream morphology. Geomorphology 33: 107-120. Yang, X. 2007. Integrated use of remote sensing and geographic information systems in riparian vegetation delineation and mapping. International Journal of Remote Sensing 28: 353-370. Young, R. G., Matthaei, C. D. and Townsend, C. R. 2008. Organic matter breakdown and ecosystem metabolism: functional indicators for assessing river ecosystem health. Journal of the North American Benthological Society 27: 605-625. Zilioli, E. and Brivio, P. A. 1997. The satellite derived optical information for the comparative assessment of lucastrine water quality. Science of the Total Environment 196: 229-245. 43