Period 4, Growth of E. coli and determination of its
advertisement

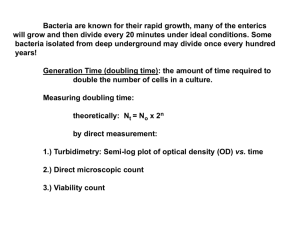
Period 4 (Feb. 12) Growth of E. coli and determination of its doubling times on enriched and minimal media. “The study of the growth of bacterial cultures does not constitute a specialized subject or branch of research: it is the basic method of Microbiology” Jacques Monod (1949) Background: Use light-scattering to follow the growth of E. coli K12 in batch culture. Grow the organism in an enriched medium (Luria Broth) and a minimal medium (N–C– glucose ammonium) and determine its average doubling time (generation time) on each of these media. We will derive and discuss the equations for determining average doubling time (see below) in discussion section and will pool and analyze your data in discussion section. Solve the two problems that follow the equations. See also pages 8890 of Schlegel (included). Materials: 1. A fully-grown culture of E. coli K12 on Luria Broth medium (5 ml/pair) 2. A fully-grown culture of E. coli K12 on N-C- medium containing glucose (0.4%) and ammonium chloride (10 mM) (5 ml/pair) 3. Luria Broth (50 ml in a sterile 250 ml flask) (2/pair, one is an extra.) 4. N–C– medium with glucose and NH4Cl (50 ml in a sterile 250 ml flask) (2/pair, one is an extra) 5. Shaking water baths at 37°C with the appropriate holders for 250 ml flasks 6. Spectronic 20’s and spectrophotometers warmed up 7. Tubes for Spectronic 20’s and cuvettes for spectrophotometers 8. Means of washing Spectronic tubes and cuvettes between readings 9. Sterile pipettes 10. Graph paper (semi-log, provided in lab manual) 11. Sterile H2O 12. Test tubes and test tube racks for taking samples Methods: 1. Dilute your E. coli starter culture 1/100 (0.5 ml to 50 ml) in Luria Broth. Incubate at 37°C with shaking (aeration). Follow growth by measuring O.D.650 (O.D. at 650 nanometers = 0.65 micrometers) with a Spectronic 20 or a Spectrophotometer. Use the same instrument throughout and decide on the appropriate blank to use throughout. Keep in mind that you have enough culture to make 8 or 9 measurements in the Spectronic 20 and that you do not want to interrupt the growth of the culture to remove more samples than that in any case. What will be the starting O.D. of your culture? How do you know? Assume that the average doubling or generation time of your culture is 30 min. At what intervals do you want to measure O.D.650? Why? Do you want the interval to be the same for all samples? Why or why not? Why do you think the culture stops growing? How would you test your hypothesis? PLEASE PLOT YOUR RESULTS ON THE GRAPH PAPER PROVIDED, IN ADDITION PMB C112L Spring 2007 Sydney Kustu 4-1 TO ANY OTHER WAY YOU PLOT THEM. IT IS BEST TO DO THIS DURING THE LAB. 2. Dilute your E. coli starter culture 1/50 (1 ml to 50) in N–C– medium containing 0.4% glucose and 10 mM NH4Cl. Perform the same manipulations as above. Assume that the average doubling or generation time of your culture is approximately 60 min. Do your answers to any of the above questions differ? Why or why not? Why do you think the culture stops growing? How would you test your hypothesis? Why do you think the average doubling time of the culture might be longer in minimal than enriched medium? Do you think the cultures on enriched and minimal media stop growing for the same reason? How would you test your hypothesis? Analysis: As you are doing the experiments, plot your results on semilog paper. When you have completed the experiments, determine the average doubling times of your cultures. From the doubling times calculate the corresponding growth rate constants µ. Design experiments to test some of the hypotheses you formulate in connection with the questions above. PMB C112L Spring 2007 Sydney Kustu 4-2 S. KUSTU BACTERIAL GROWTH BACTERIAL GROWTH Growth: Orderly increase of all chemical constituents, components I. During a period of balanced growth of a bacterial culture, an increase in biomass is accompanied by a comparable increase in all other measurable properties of the population, e. g. protein, RNA, DNA and intracellular water. Cultures undergoing balanced growth maintain a constant chemical composition. Biomass is proportional to cell numbers (n). We wish to determine the growth rate of a population of unicellular bacteria under conditions of balanced growth. II. Equations for balanced growth of a population of unicellular bacteria. Let n = number of bacteria in a fixed volume of medium. 1. dn/dt = µn; µ is growth rate constant. Intuitively appealing assumption. Can't measure instantaneous dn/dt, so Rearrange dn/n = µdt Integrate n ∫ t dn/n = no ∫ µdt to ln n - ln no = µ(t - to) no = number at time to n = number at time t Let to = 0 2. ln n - ln no = µt Convert to common logarithms using ln n = 2.3 log10 n 2a. 2.3 (log n - log no) = µt This can be rearranged to show that it is the equation for a straight line ( y = mx + b; y = ax + b) PMB C112L Spring 2007 Sydney Kustu 4-7 S. KUSTU BACTERIAL GROWTH 2b. log n = µt /2.3 + log no If we plot log n as a function of time, the slope of the resulting line is µ/2.3 and µ = slope x 2.3 Define g as the doubling time or mean generation time, that is, the time for no cells to form 2no cells. Using equation 2a. 2.3 (log 2 no - log no) = µg 2.3 (log 2 + log no - log no) = µg 2.3 x .30 = µg 3. g = 2.3 x .3/µ = 0.69/µ The units for g are hours or minutes. g is inversely related to growth rate. (shorter g = faster growth rate) From equation 2a, log n/no = µt/2.3 Taking antilogarithms n/no = 10µt/2.3 (check by going backwards and taking logs) 4. n = no x 10µt/2.3 PMB C112L Spring 2007 Sydney Kustu 4-8 S. KUSTU BACTERIAL GROWTH STUDY QUESTION Bacterial populations seldom maintain exponential growth at high rates for long. The claim is made that a single bacterium with a generation time of 20 min. would produce a population of cells weighing 4000x the weight of the earth in 48 hours. Assess this claim. (I can verify it approximately but not exactly!!) Assume: 1 cell =1 pg dry weight = 10 pg wet weight earth = 6.59 x 1021 tons I used 2000 lb/ton There are 454 g/lb PMB C112L Spring 2007 Sydney Kustu 4-9 S. KUSTU BACTERIAL GROWTH III. Growth in batch culture. A batch culture is a culture grown in a closed container with no addition of nutrients (or other substances) after the initial inoculation of bacteria. A. The exponential phase of growth is the only phase of growth that is physiologically well-defined and reproducible under given conditions. This phase begins when the growth rate reaches a constant value. Within limits, the growth rate or doubling time is determined by the environment, that is, the nature of the nutrients, the temperature, the pH, etc. B. If the growth of a batch culture is limited by the availability of a single nutrient, for example the carbon source, the yield of cells will be proportional to the concentration of this nutrient. METHODS 1. Measure: numbers, mass. Under the conditions we'll talk about they are directly related (proportional). 2. Dry weight. Isolate cells from a known volume of medium. a. Filter cells from a known volume of medium. b. Wash to remove medium components. c. Dry. d. Weigh. Insensitive: 1-5x109 cells/mg dw. Time-consuming, tedious. 3. Viable count. a. Spread known volume of culture on solid medium. b. Incubate overnight. c. Count colonies. Very sensitive: 10-100 cells/ml. Hard to get good statistics, time-consuming. 4. Light scattering O. D. α light scattered α # of bacteria/ml α dw of bacteria/ml. Usually used for routine measurements: 107/ml (106/ml with nephelometer) PMB C112L Spring 2007 Sydney Kustu 4-10 S. KUSTU BACTERIAL GROWTH PROBLEM SET 1. To determine the growth rate of a bacterial culture on a particular medium, you can measure the optical density (light scattering ability) of the culture at various times. Time (minutes) O. D. log O.D. 0 56 97 140 187 230 274 346 434 .047 = 4.7 x 10-2 .071 = 7.1 x 10-2 .111 = 1.11 x 10-1 .182 = 1.82 x 10-1 .301 = 3.01 x 10-1 .478 = 4.78 x 10-1 .731 = 7.31 x 10-1 .920 = 9.20 x 10-1 1.02 2 + .672 = -1.328 2 + .851 = -1.149 1 + .045 = -.955 1 + .260 = -.74 1 + .479 = -.521 1 + .679 = -.321 1 + .864 = -.136 1 + .964 = -.036 .009 Plot O. D. (light scattered) as a function of time. Plot log O. D. as a function of time. Calculate the growth rate constant µ. Calculate the doubling time g. 2. You want to inoculate 100 ml of a bacterial culture at 8 a. m. so that it will reach a cell density of 5 x 107 cells/ml at 11 a. m. The doubling time g of the bacterial strain is 58 minutes on the medium you are using. You have a culture containing 108 cells/ml. What volume of this culture should you use as inoculum? Assume there is no lag, that is, that the cells begin growing exponentially as soon as you inoculate. PMB C112L Spring 2007 Sydney Kustu 4-11 S. KUSTU BACTERIAL GROWTH SOLUTIONS TO PROBLEM SET Problem 1 exponential phase slope = µ/2.303 µ = slope x 2.303 µ = 2.303 [-0.56 - (-1.13)]/[180 - 60] µ = 2.303 [1.13 - 0.56]/[120] This is determined from the plot, which makes use of all the data µ = 2.303 x .57/120 = .0109/min or 60 x .0109 = 0.65/hour g = 0.69/µ = 0.69/.0109 = 63 minutes or 0.69/0.65 = 1.06 hours Problem 2 Let n = number of cells per 100 ml of culture log n - log no = µt/2.303 log (5 x 109) - log no = µ x 3/2.303; µ = 0.69/g = 0.69 hr-1/(58/60) log (5 x 109) - log no = (0.69 x 3 x 60)/(2.303 x 58) log no = log (5 x 109) - (0.69 x 3 x 60)/(2.303 x 58) log no = 9.7 - 0.92 = 8.78 no = 6 x 108 Use 6 ml inoculum = 6 x 108 cells = no PMB C112L Spring 2007 Sydney Kustu 4-12