Dalton Dalton 1808 -All matter is composed of atoms
advertisement
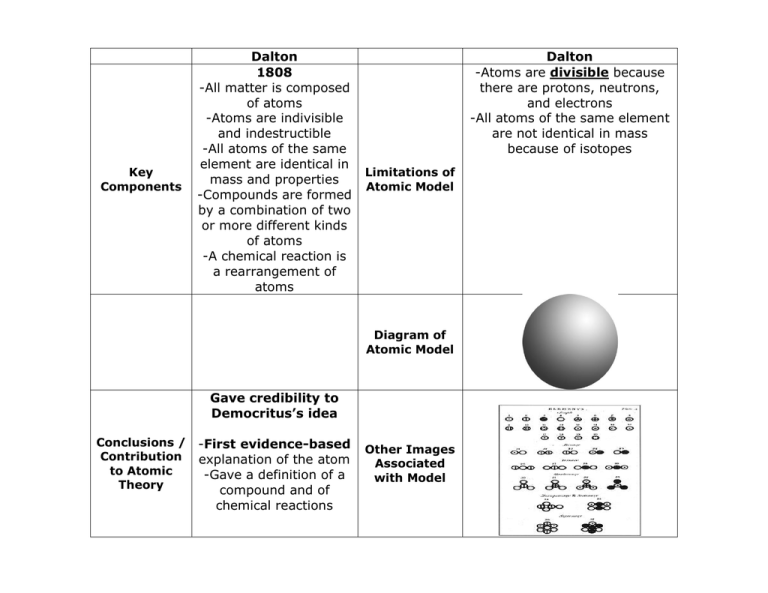
Key Components Dalton 1808 -All matter is composed of atoms -Atoms are indivisible and indestructible -All atoms of the same element are identical in mass and properties -Compounds are formed by a combination of two or more different kinds of atoms -A chemical reaction is a rearrangement of atoms Dalton -Atoms are divisible because there are protons, neutrons, and electrons -All atoms of the same element are not identical in mass because of isotopes Limitations of Atomic Model Diagram of Atomic Model Gave credibility to Democritus’s idea Conclusions / Contribution to Atomic Theory -First evidence-based explanation of the atom -Gave a definition of a compound and of chemical reactions Other Images Associated with Model Key Components Quantum 1940’s- now -Mathematical model -describes region of space (cloud) where an electron will probably be -Electron clouds are called orbitals: s, p, d, f -Only 2 electrons can be in an orbital at the same time and they must pair with opposite spin -Sometimes called the Quantum Mechanical Model Other Images Associated with Model Conclusions / Contribution to Atomic Theory Quantum -Theoretical physicists are developing theories for exceptions to the quantum model Limitations of Atomic Model Diagram of Atomic Model -Current Atomic Model Gives an atomic model based on probability and geometric shapes -Electrons have certain allowed quantum “numbers” (characteristics) -Treats electron as both a wave and a particle Other Images Associated with Model 1s, 2s, and 2p images around a nucleus Thomson Key Components 1897 Plum Pudding Model -Discovered electron using an Limitations of experiment Atomic Model involving cathode ray tube Thomson -No explanation why the protons and electrons do not attract and bond to each other - No proof of the proton just a theory at this time. -No organization to the sea of positive charges (protons) and negative charges (electrons) Diagram of Atomic Model -Unlike Dalton proved an atom is divisible due to the discovery of Conclusions / the electron. Other Images -Negative electrons Contribution Associated to Atomic are surrounded by a with Model Theory soup of positive charges -Gives subatomic particles to the atom Thomson’s Cathode Ray Tube Key Components Rutherford 1911 Gold Foil Experiment: -Fired a beam of alpha particles directly at a thin sheet of gold foil -The particles were predicted to pass straight through, but a small fraction of them bounced back because they had collided with something within the atom Limitations of Atomic Model Rutherford -No explanation for location of negative electrons other than outside the nucleus. -No explanation for why the electrons and protons are not attracting to each other. Diagram of Atomic Model Conclusions / Contribution to Atomic Theory -Proves nucleus at the center of the atom where protons are tightly packed together -Nucleus is dense (high mass in small volume) and positively charged -Rest of the atom is mostly empty space. -Came after Thomson’s Plum Pudding Model Other Images Associated with Model Top: Gold Foil Experiment Predicted Bottom: Gold Foil Experiment Results Key Components Bohr 1913 -The atoms has a small, positively charged nucleus surrounded by electrons that travel in circular orbits, similar to the planets in the solar system Bohr -Simplistic in its treatment of the electron path as a simple circle, instead of a 3D shape Limitations of Atomic Model Diagram of Atomic Model -Gave an idea of where electrons are located after Rutherford -Gives quantized energy Conclusions / levels for the orbit of Contribution electrons, which explains to Atomic why the electrons do not Theory fall into the nucleus -Explains the behavior or electron energy level transitions for a hydrogen atom model Other Images Associated with Model The emission spectrum of hydrogen showing quantized energy transitions for electrons Democritus/Aristotle 400BC Democritus: Matter is composed of “atomos” Key Components Aristotle: Basic building block of matter is composed of 4 elements: earth, wind, water, and fire. Limitations of Atomic Model Democritus vision of the atom First Idea of the Atom Most people believed Aristotle’s vision of matter. Conclusions / Contribution to Atomic Theory Aristotle’s belief of what matter is made of. Democritus/Aristotle -Neither Democritus nor Aristotle did any research or experiments to try and determine what matter is composed of. Both were philosophers.

