Cation Group II (Copper Arsenic group )
advertisement
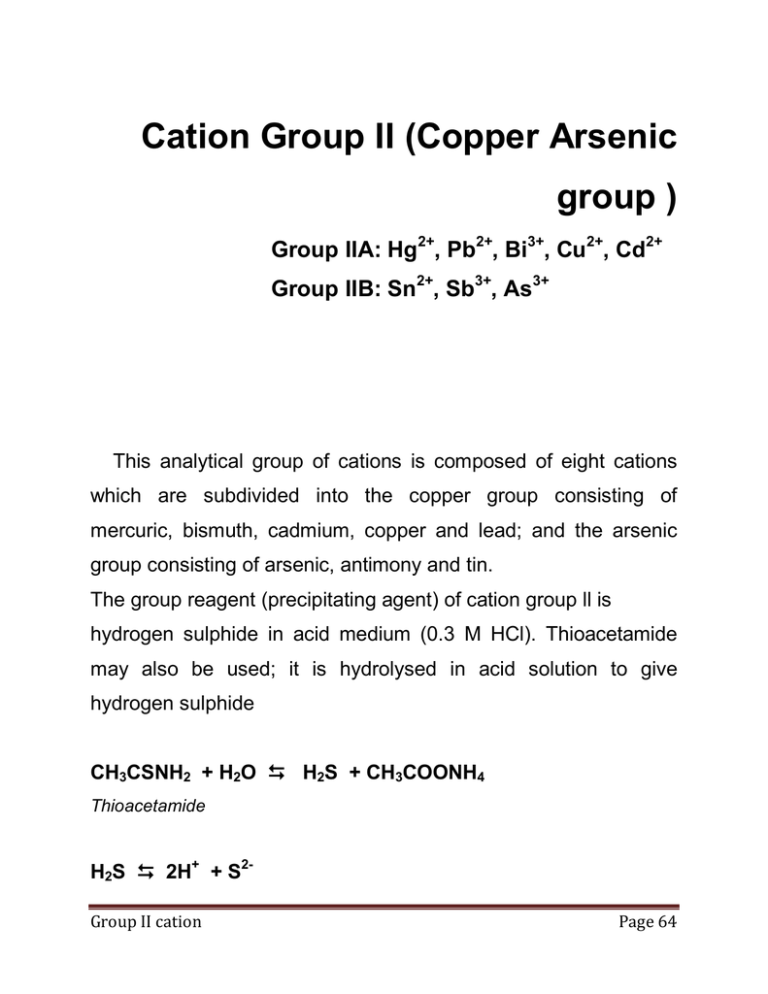
Cation Group II (Copper Arsenic group ) Group IIA: Hg2+, Pb2+, Bi3+, Cu2+, Cd2+ Group IIB: Sn2+, Sb3+, As3+ This analytical group of cations is composed of eight cations which are subdivided into the copper group consisting of mercuric, bismuth, cadmium, copper and lead; and the arsenic group consisting of arsenic, antimony and tin. The group reagent (precipitating agent) of cation group ll is hydrogen sulphide in acid medium (0.3 M HCl). Thioacetamide may also be used; it is hydrolysed in acid solution to give hydrogen sulphide CH3CSNH2 + H2O H2S + CH3COONH4 Thioacetamide H2S 2H+ + S2Group II cation Page 64 The real significance of the [H+] as a control of [S2-] can be seen by examining the ionization of H2S. K a= H + 2 [S2− ] = 1.3 x 10−20 [H2 S] A saturated solution of H2S is approximately 0.1 M; therefore, for a saturated solution of H2S, the expression may be simplified to, K a= H+ 2 [S2− ] 0.1 Or [H+]2+ [S2-] = constant H2S 2 H+ + S2HCl H+ + Clc.i.e According to the theory of common ion effect, increasing the concentration of H+ ions in solution of H2S in water, shift the equilibrium to the left, decreases the dissociation of H 2S in H2O and hence decreases the sulphide ion concentration. On the other hand, the addition of strong base shifls this equilibrium to the fight with a resultant increase the [S 2-]. Group II cation Page 65 Msl [M2+]+ [s2-] Ksp = [M2+][S2-] The metal will precipitate when the product of [M2+] and [S2-] exceeds the solubility product. This means that the limiting factor in precipitation is the sulphide ion concentration which is controlled by the pH (the hydrogen ion conc.). Therefore, to precipitate cations of group ll (having lower solubility product than those of group llIB), we need relatively small amount of sulphide ion. If the [H+] is controlled to become 0.3 M. the [S 2-] will be 1 x 10-19 M. This is sufficient to precipitate the sulphides of the elements of cation group ll. Adjustment of the acidity An increase in [S2-] by decreasing the acidity (< 6.3 M HCl) results in: a) Precipitation of sulphides of group lll. b) Dissolution of sulphides of group IIa as thio-anions (see group Ila). Group II cation Page 66 A decrease in [S2-] by increasing the acidity (> 0.3 M HCI) results in : a) Prevention of the precipitation of CdS, PbS. SnS2; (have higher solubility products). b) Formation of stable soluble complexes as[CdCl4]2-, [SnCl6]2-. [SbCl4]-. so they cannot precipitate by addition of H2S. Subdivision of cations group II intoIIA and IIB. Cation group ll contains eight elements; this large number is too difficult to analyse satisfactorily unless it is subdivided into two subgroups: group IIA (copper group) and IIB (arsenic group). The subdivision is based on the differences in the electronegativity values. Thus, the members of the copper group have low electronegativily values and are, therefore, insoluble in alkali sulphides or alkali hydroxides. On the other hand, the members of the arsenic group are sufficiently electronegative to dissolve in alkali sulphides giving thioanions, and in alkali hydroxides giving both thioanions and oxyanions (amphoteric sulphides). Group II cation Page 67 NB. : The eleclronegativity and the acidity increases by increasing the oxidation number. The thio and oxyanions of group IIB are re-precipitated on acidification acetic acid is used; since if strong HCl is used, antimony and tin sulphides will dissolve forming soluble complexes. Group II cation Page 68 Copper Subgroup Cation group II 1. Boil with H2O2 and expel the excess 2. Adjustment the acidity 3. Add H2S Residue Sulphides of Group II NaOH or Na2S Residue Centrifuge Ppt of sulphides of Soluble complexes subgroup IIA subgroups IIB of Flowchart for separation of cations group II to subgroup IIA and IIB N.B.: (1) Unless Hg2+ is an element of group IIA, but it appears in bothIIA and II B subgroups because its precipitate HgS in group Group II cation Page 69 IIA is partially soluble in alkali sulphide (Na2S) and pass with group IIB HgS + Na2S Hg(SNa)2 soluble salt (2) Stannous sulphide is not amphoteric so, it is incompletely soluble in either NaOH or Na2S. Therefore, to separate stannous with the subgroup IIB cation sulphides, we have to oxidize stannous to stannic (Sn4+) by boiling with hydrogen peroxide. The stannic (Sn4+) has higher oxidation number and higher electronegativity). Sn2+ + H2O2 𝑯𝑪𝒍 Sn4+ + H2O The excess H2O2 must be decomposed by boiling, otherwise it will oxidize the H2S reagent to colloidal sulphur which is difficult to separate 2 H2O2 𝑺𝒕𝒓𝒐𝒏𝒈 𝒃𝒐𝒊𝒍𝒍𝒐𝒊𝒏𝒈 2 H2O + O2 H2O2 + H2S So + 2 H2O colloidal sulphur Group II cation Page 70 Copper subgroup (Cation group II A) Hg2+, Pb2+, Bi3+, Cu2+, Cd2+ HgS, PbS, Black ppt CuS, Bi2S3, Brown ppt CdS Yellow Adjustment the acidity Add H2S Heat with 1:1 HNO3/H2SO4 Residue Centrifuge Pb2+, Bi3+, Cu2+, Cd2+ HgS, HgO Ethanol/ H2SO4 Dissolve in aqua regia Test for Hg2+ 1) SnCl2 2) NH4OH 3) KI Dissolve in ammonium acetate Test for Pb2+ 1- Acetic acid +K2CrO4 2- KI Test for Bi3+ 1- Xss H2O 2- Sodium stannite Test of Cu2+ in presence of Cd2+ 1) Acetic acid + ferrocyanide 2) KI Centrifuge Residue Bi3+, Cu2+, Cd2+ PbSO4 White ppt Conc. NH4OH Residue Bi(OH)3 white gelatinous ppt dissolve in HCl Centrifuge [Cd(NH3)4 ]2+ colourless [Cu(NH3)4]2+ blue Test for Cd2+ In presence of Cu2+ KCN + H2S Flowchart for analysis of cation group IIA Group II cation Page 71 Mercuric (ll) Ion (Hg2+) Previously discussed with cation group I Bismuth (Ill) ion (Bi3+) Reactions Important in the Separation and Identification of Bismuth (Bi3+) 1. Group reagent Bi 3+ + H2S 𝟎.𝟑 𝑴 [𝑯+] Bi2S3 + H+ dark brown 2. Dissolution Oxidation of sulphides BiS3 + HNO3 Bi3+ + So + NO + H2O Complex formation reaction BiCl3 + conc. HCl [BiCl4]bismuth te-trachioride soluble complex 3. Confirmatory Test: 1- Reaction with conc. NH4OH Bismuth is separated from Cu2+ and Cd2+ by precipitation with Group II cation Page 72 conc. NH4OH The precipitate is insoluble in excess of the reagent (no ammine complex). Bi3+ conc. NH4OH [BiCl4]white gelatinous We dissolve this precipitate in HCI Bi(OH)3 + HCl BiCl3 + H2O Two tests for bismuth by: Oxysalt formation Addition of large excess water will give white turbidity soluble in excess acid by common ion effect of the H+ BiCl3 + H2O BiOCl + 2 H+ soluble large white turbidity excess bismuth oxyehioride BiOCl + HCI BiCl3 + 2H+ or HNO3 soluble Sodium Stannite Test Bi3+ + [HSnO2]Stannite reagent Group II cation Bi° + [Sn(OH)6]2- black bismuth metal stannic hexahydroxide soluble complex Page 73 This is an oxidation-reduction reaction where the bismuth ion is reduced to metallic bismuth (black precipitate) and the stannous ion is oxidized to stannic N.B.: Sodium stannite reagent must be freshly prepared. SnCl2 + 2 NaOH Sn(OH)2 Stannous hydroxide Sn(OH)2 + xss NaOH [HSnO2]- or [Sn(OH)4]2stannite On standing (by time), the sodium stannite reagent under goes self oxidation-reduction and black precipitate of tin is formed. [Sn(OH)4]2 𝒃𝒚 𝒕𝒊𝒎𝒆 𝒂𝒖𝒕𝒐𝒙𝒊𝒅𝒂𝒕𝒊𝒐𝒏 Sno + [Sn(OH)6]2- black 4. Reaction with NaOH the bismuth hydroxide is formed which is insoluble in excess NaOH (not amphoteric) Group II cation Page 74 Bi3+ + NaOH Bi(OH)3 xss NaOH Insoluble Copper (II) ion (Cu2+) Copper (I) and copper (II) are known in solid compounds. Copper(ll) is the only common species in aqueous solution. The Cu2+ ion in solution is blue in colour, while the anhydrous cupric salts are white. In acidic solution, Cu+ and Cu2+ are related by a redox disproportionation equilibrium like that of the mercury. 2Cu+ Cu (s) + Cu2+ [𝐂𝐮+ ]𝟐 𝐊= = [𝐂𝐮𝟐+ ] At equilibrium the concentration of Cu2+ is always (1.4 x 106) [Cu+]2 . The volatile compounds of some elements give characteristic colors when the compound or its solution is exposed to a flame. Group II cation Page 75 This usually is done by dipping a clean platinum wire into the compound or its solution and holding we wire ine the oxidizing part of a Bunsen burner flame. Copper (II) nitrate gives a bright blue flame color; copper (ll) chloride gives a green flame color. Reaction Important in the Separation and Identification of Copper (Cu2+): 1. Group Precipitation Cu 2+ + H2S 𝟎.𝟑 𝑴 [𝑯+] CuS + 2H+ black 2. Dissolution : Oxidation of sulpide reaction CuS(s) + NO3-(aq) Cu2+(aq) + SO42-(aq) + NO(g) Complex formation reaction o With NH4OH Cu2+ + 4 NH4OH [Cu(NH3)4]2+ + 4 H2O blue: copper tetramine (soluble complex) Group II cation Page 76 o With KCN Cu2+ + KCN [Cu(CN)4]3cuprocyanide complex (tetracyanocuprous complex) stable complex. KCN is called masking reagent 3. Confirmatory Tests: Cu2+ + 4 NH4OH [Cu(NH3)4]2+ + 4 H2O deep - blue colour Cu2+ + 2 SCN Cu(SCN)2 thiocyanate black Cu2+ + KI Cu2I2 white cuprous iodide Cu2+ + [Fe(CN)6]4- 𝑯𝑨𝑪 + I2 brown solution Cu2 [Fe(CN)6] chocolate Group II cation Page 77 N.B. To carry out the test with ferrocyanide on the soluble copper ammine complex we have to acidify first with dil. acetic acid (until the blue colour disappear) to decompose the copper complex, and liberate the free Copper ion in the medium. [Cu(NH3)4]2+ + dil. 4 CH3COOH Cu2+ + CH3COONH4 Reaction with NaOH xss NaOH insoluble (not amphoteric) Cu2+ + NaOH 𝑯𝑨𝑪 Cu2 (OH)2 blue CuO Black Group II cation Page 78 Cadmium II ion,Cd2+ Reaction Important in the Separation and identification of cadium (Cd2+) Group precipitation and Confirmatory Test Cd 2+ + H2S 𝟎.𝟑 𝑴 [𝑯+] CdS + 2H+ Cadmium sulphide Canary yellow Dissolution : By Oxidation of sulphide CdS + dil. HNO3 Cd2+ + NO + H2O + So Complex Formation With NH4OH Cd2+ + 4 NH4OH Cd(NH3)4]2+ + NO + H2O + So cadium tetramine complex Group II cation Page 79 with conc. HCI Cd+ + conc. HCl [CdCl4]2with KCN Cd2+ + 4 KCN [Cd(CN)4]2cadium tetracyanide unstable complex N.B. How can you separate and identify a mixture of CdS, CuS ? Group II cation Page 80 Arsenic subgroup The elements of this group are arsenic, antimony, and tin As3+, As5+ , Sb3+ , Sb5+ , Sn2+ Airsenious, Arsenic , Antimonous ,Antimonie ,Stannous When group II is boiled with H2O2, the acidity is adjusted at 0.3 M HCl and H2S or thioacetamide is added, the following amphoteric sulphide precipitates of subgroup IIB are formed. [As2S3 , As2S5 ], [Sb2S3 , Yellow ppt Sb2S5 ], orangeppt SnS2 brown ppt Because of their high electroneganvities, their sulphides (except SnS) are amphoteric. These precipitates are soluble in NaOH (separation of subgroups IIA & IIB) (or Na2S) producing a mixture of thio- and oxysalts (centrifugate in the separation of subgroup cation precipitate of IIA & IIB). Group II cation Page 81 As2S3 + NaOH AsS2- + AsO2- thioarsenite As2S5 + NaOH AsS3- oxyarsenite + AsO3- thioarsenatc Sb2S3 + NaOH SbS2thioantimonite Sb2S5 + NaOH SbS3- oxyarsenate + SbO2oxyantimonite + SbO3- thioantimonate oxyantimonate SnS + NaOH sparingly soluble SnS2 + NaOH [SnOS22-]2Thio oxystannate N.B. : Re-precipitation of the Arsenic Group Sulphides The alkaline solution, containing the ions of the arsenic group in the form of soluble complex thio- or oxy-anions, is acidified to Group II cation Page 82 destroy these complex ions and to re-precipitate the sulphides of the arsenic group. 3 AsS3- + AsO3- + 4H+ 2 As2S3 + 2 H2O 5 AsS3- + AsO3- + 6H+ 2 As2S5 + 3 H2O 3 SbS2- + SbO2- + 4H+ 2 Sb2S3 + 2 H2O 5 SbS3- + SbO3- + 6H+ 3 Sb2S5 + 3 H2O SnOS22- + 2H+ SnS2 + H2O In acidification of the previous soluble complex solutions, dilute acetic acid is preferred to HCl. As HCl will form soluble complexes with Sn4+ and Sb3+, so prevent their reprecipitation as sulphide salt (are lost with the discarded centrifugate.) Example SbS2- + SbO2- + conc. HCl [SbCl4]SnOS22- + conc.HCl [SnCl4]2soluble complex Group II cation Page 83 Centifugate of Cation Group II acidify with acetic acid add H2S Residue As2S3 , Yellow ppt As2S5 , Sb2S3 orange ppt Sb2S5 , SnS2 brown ppt Centrifuge Discard Heat with 1:1 HCl Just before boiling Residue Centrifuge As2S3 , SbCl3 , SnCl4 , As2S5 , Heat to dissolve alkalinization in conc. HNO3 Test for arsenic 1. Ammonium molybdate reagent 2. Magnesia mixture reagent 3. Battendroff reagent with NH4OH Test for stannic in presence of Sb3+ (boil with conc, HCl and iron wire Test for Sb in presence of sn 1. Oxalic acid + H2S 2. Acetic acid and solid sodium thiosulphate 3. Excee water Flowchart for analysis of cation group IIB Group II cation Page 84 Arsenious (Ill) ion and Arsenic (V) ion, As3+, As5+ Reactions Important in the Separation and Identification of As3+, As5+ 1. Group precipitation 2As 3+ 2As 5+ + 3 H2S + 5 H2S 𝑯+ 𝑯+ As2S5 + 6H+ As2S5 + 10 H+ yellow 2. Dissolution by: A- Complex formation: (1) Alkali sulphide (Na2S) As2S3 + S2- AsS2 As2S5 + S2- AsS3(2) Alkali hydroxide (NaOH) As2S3 + NaOH AsS2- + AsO2- As2S5 + NaOH AsS3- + AsO3- Group II cation Page 85 (3) Yellow ammonium sulphide (Poly ammonium sulphide) (NH4)2Sx it is mixture of ammonium sulphide and sulphur. The later has an oxidizing power. All the products of the dissolution will give the soluble complex of the higher oxidation state, (thio and oxy arsenate) As2S3 + (NH4)2Sx AsS3- + (NH4)2Sx-1 As2S5 + (NH4)2Sx B- Oxidation by conc. HNO3 As2S3 + conc. HNO3 H3AsO4 As2S5 + conc. HNO3 4. Separation and Confirmatory Tests The arsenious sulphide and arsenic sulphide are insoluble in l2 M HCl (differ from sulphides of antimony and tin), but by boiling they are partially soluble. Group II cation Page 86 To confirm the presence of arsenic several tests can be done. 1- Gutziet Test The arsenic sulphide is dissolved by conc. HNO3 and treated with zinc dust in acid medium (strong reducing agent). A filter paper moistened with AgNO3 is exposed to the arsine gas produced from the previous reaction, Blackening to the filter paper occurs. . H3AsO4 + Zno /H+ AsH3 + Zn2+ arsine gas ASH3 + AgNO3 Ago + H3AsO3 black If Sb3+ is present it will give the same reaction with the production of stibine gas which also reduced AgNO3 to Ag°. Flietmann Modification To prevent the interference of Sb3+ in the test, we use zinc dust in alkaline medium or aluminium in alkaline medium (mild Group II cation Page 87 reducing agent) which can reduce the arsenic and not the antimony. 2- Ammonium molybdate Test ∆ 𝒄𝒐𝒏𝒄.𝑯𝑵𝑶𝟑 (NH4)3AsO4.12MoO3 H3AsO4 + (NH4)8MoO4 canaly yellow ammonium arsenomolybdate 3- Magnesia mixture Test Mixture of (NH4OH, NH4Cl, MgCl2) H3AsO4 + NH4+ + Mg2+ MgNH4AsO4 white crystalline ppt magnesium ammonium arsenal 4- Battendroff test The stannous chloride in conc. l-lCl, reduces arsenic salts to metallic arsenic Group II cation Page 88 H3AsO4 + SnCl3 + HCl Aso + SnCl62- + H2O It is an oxidation-reduction reaction. Antimonous (III) ion and Antiomnic (V) ion (Sb3+, Sb5+) Reaction Important in the separation and Identification of Sb3+, Sb5+ Group precipitation 2 Sb 3+ 2 Sb 5+ + 3 H2S + 3 H2S 𝑯+ 𝑯+ Sb2S3 + 6 H+ Sb2S5 + 10 H+ orange Dissolution by complex formation 1- Alkali sulphide (Na2S) Sb2S3 + S2- SbS2 Sb2S5 + S2- SbS3- Group II cation Page 89 2- Alkali hydroxide (NaOH) Sb2S3 + NaOH SbS2- + SbO2 Sb2S5 + NaOH SbS3- + SbO33- Yellow ammonium sulphide (NH4)2Sx Sb2S3 + (NH4)2Sx SbS3- + (NH4)2Sx-1 Sb2S5 + (NH4)2Sx 4- Conc. HCl Sb2S3 + conc. HCl SbCl4- + H2S Sb2S5 + conc. HCl SbCl6- + H2S Separation and Confirmatory test: 1- Oxysalt formation SbCl3 + H2O SbOCl + H+ large excess Group II cation white turbidity antimony oxychlotidc basic salt Page 90 The reaction is reversed by adding HCl SbOCl + HCl or HNO3 SbCl3 + H2O soluble (common ion effect of H+) 2- Sodium thiosulphate Test Sb3+ + S2O32- + H+ SbOS2 solid orange antimony oxysulphide 3- Iron wire Test Sb 3+ o + 3 Fe 𝑯+ Sbo + 3 Fe2+ black deposit Oxidation-reduction reaction 4- Test for Sb3+ in presence of Sn4+ Here we test for Sb3 by H2S to give orange ppt. But the Sn4+ interferes and gives the brown ppt. of SnS2. To get rid of this interference, we add oxalic acid which forms two complexes with Sb3+ and Sn4+, The complex with Sn4+ is stable, while that with Sb3+ is unstable, so on passing H2S, orange ppt. of Sb2S5 is formed. Group II cation Page 91 Sn4+ [Sn(C2O4)3]2- + stable Sb3+ [Sb(C2O4)3]3- + oxalate unstable H2S Sb2S3 orange Oxalic acid is called masking agent. Stannous (II) ion, Stannic (IV) Ion (Sn2+,Sn4+). Reaction Important in the Separation and Identification of Sn2+,Sn4+. Group precipitation Sn2+ + H2O2 + 2H+ Sn4+ + 2 H2O Sn 4+ + H2S 𝑯+ SnS2 brown Group II cation Page 92 Dissolution by complex formation 1- Alkali sulphide (Na2S) SnS + S2- sparingly soluble SnS2 + S2- SnS322- Alkali hydroxide (NaOH) SnS + NaOH SnS2 + NaOH sparingly soluble SnoS22- Thio oxystannate complex 3- Yellow ammonium sulphide (NH4)2Sx SnS + (NH4)2Sx SnS32- + (NH4)2Sx-1 SnS2 + (NH4)2Sx 4- Conc. HCl SnS + conc. HCl [SnCl4]2 SnS2 + conc. HCl [SbCl6]2- Group II cation Page 93 Separation and Confirmatory Test Mercuric chloride test: We have to reduce the Sn4+ to Sn2+ using iron wire in acid medium. Then HgCl2 oxidizes Sn2+ to Sn4+ and being reduced according to the amount of HgCl2 used. The test may give white ppt. (HgCl2) or gray ppt. (Hg2Cl2+ Hgo) or finally black ppt. (Hgo) (oxidation-reduction reaction). Sn4+ + Fe° + H+ Sn2+ + Fe2+ (iron wire/H+) white Sn2+ + HgCl2 Hg2Cl2 + [SnCl6]2white Hg2Cl2 + xxs HgCl2 Hgo + [SnCl6]2Black 4- Test for stannic in presence of Sb3+ We have to boil with iron wire (Fe°) in acid medium for two reasons; Sb 3+ o + 3 Fe 𝑯+ Sbo + 3 Fe2+ removed by fiitration Sn Group II cation 4+ o + 3 Fe 𝑯+ Sn2+ + 2 Fe2+ Page 94





