Untitled
advertisement

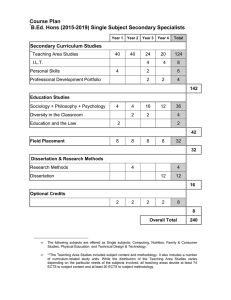
1 .................................................................................................................................... 3 ....................................................................................... 3 ..................................................................................................................................... 3 ............................................................................................................... 4 .............................................................................................................. 5 .................................................................................................................................. 7 .......................................................................................................................................... 7 ...................................................................................................................... 8 ........................................................................................................... 9 ........................................................................................ 10 ................................................................................................................................................ 11 ......................................................................................................... 12 ................................................................................................................................. 13 ............................................................. 13 ........................................................................................................................... 13 .................................................................................................................................... 16 ....................................................................................................................................... 16 ......................................................................................................................... 16 2 3 4 5 6 7 8 9 10 11 12 → 13 14 15 16 Master’s Programme in Drug Discovery and Development 120 ECTS (for the academic year 2015-2016) Supplementary studies Supplementary Book Exam on Pharmacology 5 ECTS Supplementary Book Exam on Anatomy and Physiology 6 ECTS Supplementary Book Exam on Histology and Cell Biology 5 ECTS ÅA_1902 Laboratory Basics 2 ECTS First year Principles of drug discovery and development science 8 ECTS (Contact person: Markku Koulu) Drug regulatory science 4 ECTS (Contact person: Markku Koulu) Bioinformatics in drug discovery 5 ECTS (Contact person: Sanna Soini and Harri Härmä) Therapy areas in drug discovery and translational medicine 12 ECTS (Contact person: Markku Koulu) Methods in experimental pharmacology 4 ECTS (Contact person: Sanna Soini) Computer aided drug design 4 ECTS (Contact person: Tiina Salminen, ÅA) Clinical trial design and clinical drug development 5 ECTS (Contact person: Petri Vainio and Risto Huupponen) Drug development learning project 10 ECTS (Contact person: Markku Koulu and Ullamari Pesonen) Bioentrepreneurship 5 ECTS (Contact person: Kaisu Paasio) Elective studies 8 ECTS Second year Master’s thesis research project and seminars 35 ECTS (Contact person: Markku Koulu and Sanna Soini) Master’s thesis research report (pro gradu-report) 20 ECTS (Contact person: Markku Koulu and Sanna Soini) 17 Supplementary studies Supplementary studies of maximum 60 ECTS may be necessary depending on the courses the students have passed during their previous studies. These supplementary studies are additional and will not be included in the Master´s degree. The aim of these supplementary studies is to bring everyone to approximately same level in biosciences and other topics that are relevant for the studies in Drug Discovery and Development programme. Personal study plan will be made for every newly admitted student and required studies will be defined in the study plan. Laboratory basics is a mandatory course for everyone whose BSc degree is not awarded in Finland. DRUG0001 Supplementary Book Exam on Pharmacology 5 ECTS Rang, Dale, Ritter, Flower, Henderson: PHARMACOLOGY, Elsevier ISBN-13 978-1-4377-1933-8 (International Edition). DRUG0002 Supplementary Book Exam on Anatomy and Physiology 6 ECTS Tortora GJ, Derrickson B: PRINCIPLES OF ANATOMY AND PHYSIOLOGY, Maintenance and Continuity of the Human Body, International Student Version (13th Edition), selected pages. DRUG0003 Supplementary Book Exam on Histology and Cell Biology 5 ECTS Kierszenbaum AL, Tres LL: HISTOLOGY AND CELL BIOLOGY, An Introduction to Pathology (3rd Edition), selected pages. ÅA_1902 Laboratory Basics 2 ECTS (Flexible JOO-study right needed) Subject: Cell Biology Persons in Charge: Diana Toivola, Annika Meinander Objectives The course is a bridging course for students in MSc programme in Biomedical Sciences. The course will teach students basic laboratory techniques, including pipetting, weighing, pH measurements and good laboratory practice. Students will also learn basic laboratory safety issues. Modes of Study Lectures, demonstrations, laboratory work. The course will have exam(s), and reports on the laboratory work. Evaluation Pass/fail Previous Studies 18 BSc in biofield and accepted to the MSc programme in Biomedical Imaging or MSc programme in Drug Discovery and Development. Recommended Year of Study 1. Year Study Materials Moodle 19 DRUG005 Principles of Drug Discovery and Development (8 ECTS) Learning outcomes The aim of the study module is that students can describe the purpose of the different phases of the drug discovery and development process and discuss which particular aspects are emphasized at each stage. Students can critically evaluate and select appropriate research methods to elucidate the pharmacological and pharmaceutical properties of drug molecules as well as to assess the toxicological and safety profiles of drug candidates during the various stages of non-clinical and clinical drug development. Furthermore, students should demonstrate that they understand differences that exist between “conventional” small molecule drugs and biotechnologically produced biological drugs in terms of their chemical, biological and pharmacological properties that give rise to to rather different drug development processes. In addition, students should be able to explain the role of intellectual property rights (IPR) in drug development and how this is reflected in the strategies of drug companies. Students should also become familiar with the ethical guidelines that regulate the use of laboratory animals in research and clinical investigations carried out in humans. Students will also be able to critically assess some historical cases of drug development and to compare these with contemporary cases in order to obtain a wider perspective of the progress that has taken place in drug development science. As built-in component, students practice communication skills by preparing scientific presentations. Contents This study module consists of topics divided into the following areas: a) general introduction to the principles of drug discovery and development (historical perspectives and future trends in drug discovery and development, general role of drugs in health care and society, the roles of academia and drug companies in the different phases of drug development, regulatory aspects in drug development), b) drug discovery (identification of biological targets and their validation, screening assay development, high-throughput screening (HTS), hit validation, role of medicinal chemistry in drug discovery, computer aided drug design and lead optimization, methods of production of biological drugs), c) non-clinical drug development (animal efficacy and dosing studies, characterization of pharmacokinetic properties of drug molecules (ADME), characterization of mechanisms of action, toxicological screening and safety evaluation, use of animal models of diseases and translational medicine in drug development), d) clinical drug development (first-inman studies, pharmacodynamic and pharmacokinetic studies in man, clinical phases of drug development, clinical trial design), e) seminars on cases of some historical and contemporary drug discovery and development projects, f) journal club and g) lab work. Teaching methods Lectures, seminars, journal club, lab work Teaching language English Modes of study Available for degree programme students. Evaluation and evaluation criteria Numerical grade (0-5). Evaluation criteria are based on: a) knowledge of the entire area of the study module, as measured by a written examination (70 % of the total grade), b) seminar and journal club presentations and activity (20 % of the total grade) and c) written report of the lab work (10 % of the total grade). Recommended year of study First year of Master’s studies. Autumn semester. 20 Study materials Lecture materials, study materials for seminars and journal clubs, international databanks and guidelines regulation drug development and patent databanks. Relevant reviews and scientific articles. 21 DRUG0006 Drug regulatory science (4 ECTS) Learning outcomes The aim of the study module is that students can understand and explain the role of regulatory authorities in the drug development and approval process and that they know the legal framework that regulates the field. Students are able to describe the general outline of the procedures applied to marketing authorization applications (MAA) in the European Union and in the USA, and discuss the actions executed by the main regulatory agencies (EMA and FDA). Students are familiar with concepts such as “summary of product characteristics”, “orphan drug status”, “accelerated application” and “scientific advice”. Furthermore, students can describe how the different sections of MAAs are organized according to the common technical document (CTD) format and how data are presented to demonstrate the pharmaceutical quality, efficacy and safety of a new drug. Students can describe methods used in pharmacovigilance in order to collect safety data of drugs that are already on the market. Furthermore, students can design and analyze regulatory toxicological and safety studies required of a new drug molecule. In addition, students will be able to critically analyze interesting regulatory science cases. As built-in component, students practice communication skills by preparing scientific presentations. Contents This study module consists of topics divided into the following areas: a) role and activities of drug regulatory authorities in the European Union and in the USA (legal frameworks, guidelines, procedures used in the marketing authorization application, European Medicines Agency (EMA), Food and Drug Administration (FDA), Finnish Medicines Agency (FIMEA), common technical document format (CTD), pharmacolovigilance activity), b) regulatory toxicological and safety evaluation studies (acute, subacute and chronic toxicity, genotoxicity and mutagenicity, carcinogenicity, reproductive toxicity, environmental toxicity of drugs and their manufacturing procedures, common safety studies), c) historical cases in drug regulatory science (e.g. thalidomide, clozapine, COX2-inhibitors, terfenadine, Pandemrix). Teaching methods Lectures, “meet-the-expert” lectures, seminars, lab work Teaching language English Modes of study Available for degree programme students. Evaluation and evaluation criteria Numerical grade (0-5). Evaluation criteria are based on: a) knowledge of the entire area of the study module, as measured by a written examination (70 % of the total grade), b) seminar presentations and activity (20 % of the total grade) and c) written report of the lab work (10 % of the total grade). Recommended year of the study First year of Master’s studies. Autumn semester. Study materials Databases available on web pages of FIMEA, EMA and FDA. ICH guidelines. Relevant review articles and scientific reports. 22 DRUG0007 Bioinformatics in Drug Discovery (5 ECTS) Learning outcomes By the end of the study module students will be able to a) describe how theoretical approaches can be used to model and analyse complex biological systems b) demonstrate some practical and hands-on experience with common bioinformatics tools and databases including command-line scripting c) select a suitable software tool for a given analysis or data management task d) manage and organize datasets using different programs e) analyse, visualise and interpret various types of data including nucleotide and amino acid sequence data, gene expression data and small molecule screening data f) visualise and interpret measured protein inhibitor data and calculate parameters used typically in high throughput screening g) discuss and critically evaluate statistical methods for handling and analysing data h) understand basic concepts of graphical user interfaces Contents This study module covers the following topics: Biological databases (theory and application/queries). Bioinformatics tools in drug development and commonly used software and computing environments (e.g. R, SPSS, Excel, Chipster etc.) Analysis of microchip, protein inhibitor and sequence data (e.g. gene expression arrays, RNASeq, variant analysis). Teaching methods Classroom lectures and distance learning, independent work, supervised hands-on computer classes, supervised tutorials Teaching language English Modes of study Lectures, participation and exercise sessions Evaluation and evaluation criteria Active participation in the seminars is required to pass. Some tasks require written reports. Electronic examination. Recommended year of study First year of Master’s studies. Autumn semester. Study materials Materials and handouts from the lectures and demonstrations. 23 DRUG0008/0009 Therapy areas in drug discovery and translational medicine (12 ECTS) Learning outcomes The aim of the study module is that students will be able to describe pathophysiological disease mechanisms, diagnostic methods and biomarkers as well as clinical symptoms of diseases in some main therapy areas, such as disorders of the central nervous system (CNS), cancer and oncology, endocrine and metabolic diseases, cardiovascular diseases and immune system disorders. Students must be able to discuss the mechanisms of action of currently used drugs and to critically assess their utility in the treatment of these diseases; they should also understand existing unmet medical needs. Students can explain how animal disease models are produced and employed in the development of new drugs and how translational medicine approaches are used to facilitate the entry of drug candidates into clinical development. In addition, students will be able to analyze patent databases and other sources of information in order to get insight into emerging new drug targets and drug development pipelines in each therapy area. As built-in component, students practice communication skills by preparing scientific presentations. Contents As the entire study module is based on self-guidance of students and group work, only the main topics are given in advance. These include topics from the CNS disease area (such as Alzheimer’s disease, Parkinson’s disease, depression, addiction, psychosis, multiple sclerosis), endocrine and metabolic diseases (such as diabetes, metabolic syndrome and obesity, dyslipidemias, osteoporosis), cancer and oncology (such as breast cancer, prostate cancer), cardiovascular diseases (such as heart failure, coronary heart disease) and autoimmune diseases (such as rheumatoid arthritis, psoriasis). Teaching methods Students are divided into groups of four or five. Each group will prepare a presentation on the topic of the week, and the groups will present their work in random order at the end of the week. The presentations will have the following subheadings: pathophysiological mechanisms, diagnostic criteria, clinical symptoms, current drug therapy, animal models, emerging new targets and an overview of the pipeline situation. Teaching language English Modes of study Available for degree programme students. The study module is divided into two parts: 1. part (CNS disorders; 6 ECTS) during the autumn semester and 2. part (endocrinological and metabolic disorders, cardiovascular diseases and autoimmune diseases; 6 ECTS) during the spring semester. Evaluation and evaluation criteria Numerical grade (0-5). Evaluation criteria are based on: a) knowledge of the entire area (based on the given list of topics) of the study module, as measured by a written examination (60 % of the total grade), b) seminar presentations and activity (40 % of the total grade). Recommended year of study First year of Master’s studies (autumn and spring semesters). Study materials Relevant chapters of textbooks of pathology, internal medicine, endocrine, neurology and immunology. Recent review articles of the topics. Patent databases, pipeline databases of drug discovery companies. 24 DRUG0010 Methods in experimental pharmacology (4 ECTS) Learning outcomes By the end of the study module students will be able to a) describe appropriate experimental pharmacological techniques applied in the research and drug development b) select, plan, apply and evaluate a relevant experimental system to test experimental hypotheses (e.g. in vitro or in vivo; animal species). c) analyse data and interpret results of experiments in a proper format of written lab report d) interpret and discuss the results Contents This study module covers the following topics a) physiological regulators of vascular tone, arterial blood pressure and smooth muscle cells b) smooth muscle tissue preparations (e.g. isolated ileum and aorta) c) cardiovascular diseases d) studying inotropic and chronotropic effects of drugs in vitro using isolated atrial preparations e) planning and implementation of behavioral studies with experimental animals ( e.g. animal welfare, controls, handling the animals, administration of drugs, identification of the drug effects) Teaching methods Lectures, demonstrations and lab work Teaching language English Modes of study Lectures, participation to lab work and writing lab reports with summary statistics and graphs, writing an application for a project licence for an animal experiment Evaluation and evaluation criteria Approved lab reports Study materials Materials and handouts from the lectures and demonstrations. Recommended reading Rang & Dale’s Pharmacology and Goodman and Gilman’s The Pharmacological Basis of Therapeutics 25 ÅA213017 Computer Aided Drug Design (4 ECTS) (Flexible JOO-study right needed) Person in charge Tiina Salminen General description This course is arranged by Åbo Akademi University. See http://www.abo.fi/institution/en/coursesbiosci Learning outcomes Gives an understanding of how drugs interact with macromolecules and strategies for how this information can be used in designing novel therapeutics using computational methods. Contents Drug-protein interactions, protein surface properties, small molecule database browsing programs, surface display programs and docking programs. Teaching methods Lectures, demonstrations and lab work Teaching language English Modes of study Lectures, exercises, project work, exam Evaluation and evaluation criteria Numerical grade (0-5). Study materials All notes and materials provided. Hans-Dieter Höltje, Wolfgang Sippl, Didier Rognan, and Gerd Folkers (2003) Molecular modeling: basic principles and applications. 26 DRUG0011 Clinical trial design and clinical drug research (5 ECTS) Learning outcomes The student will be able to describe the phases of clinical drug development and the prerequisites for conducting clinical trials as it comes to legislation, regulation and regulatory guidance. The student will be able to evaluate the ethical issues related with clinical trials. The student will identify the roles of the members of multiprofessional team and those of different interest parties of clinical trials. The student will be able to compile a clinical trial protocol compliant with applicable regulations and guidance and to collect and compose documents needed to initiate and execute a clinical drug trial. The student will be acquainted with statistical approach to clinical trials and selecting appropriate testing procedures for typical trial designs. The student will be able to evaluate publications based on clinical drug research. Contents Phases and types of clinical drug trials, ethical and regulatory evaluation of trials, GCP regulations and legislation regarding clinical drug research, data collection and management in clinical drug trials, composing trial protocol, monitoring and auditing clinical trials, statistical planning, reporting and interpretation of results, clinical studies for new chemical entities. Teaching methods Lectures, demonstrations of biostatistics, journal clubs, practical work: compilation of a GCP-compliant research plan for a clinical drug trial. Teaching language English Modes of study Available for degree programme students Evaluation and evaluation criteria Numerical 0-5. After passing the study module, the student should: 1) know how to make a research plan for a clinical drug study 2) know the optimal structure of a clinical drug study and most relevant restrictions and sources of errors in studies 3) know the basic principles of GCP and the central legislation and regulatory guidance for clinical drug research 4) understand the tasks of different professional groups in the efficient and safe implementation of a clinical drug study Study materials Book: Neuvonen PJ, Backman JT, Himberg J-J, Huupponen R, Keränen T, Kivistö KT (toim): Kliininen farmakologia ja lääkehoito, Kandidaattikustannus Oy 2011, pp. 965-1015 – or equivalent Relevant legislation: Directive 2001/20/EU Regulation EU No 536/2014 Directive 2005/28/EU Acts: L 488/1999, L 523/1999, A 986/1999, A 841/2010 Relevant regulatory guidance: 27 ICH guidelines: E8 General Considerations for Clinical Trials, E3 Structure and Content of Clinical Study Reports and E6 Good Clinical Practice, available on www.turkucrc.fi), other course material Detailed guidance on the collection, verification and presentation of adverse event/reaction reports arising from clinical trials on medicinal products for human use (‘CT-3’) EMA Guideline on Strategies to identify and mitigate risks for first-in-human clinical trials with investigational medicinal products. Finnish Medicines Agency Administrative regulation 2/2012 Clinical trials on medicinal products 28 DRUG0012 Drug development learning project (10 ECTS) Learning outcomes The aim of the study module is, by applying a virtual drug discovery and development project, to practice and integrate all aspects of drug development from (a) evaluation of biological significance of the given target protein and its possible role in the pathophysiology of diseases to (b) design of entire development program for the virtual drug molecule. Students prepare first an overview of the target and possible indication (disease), utilize patent databases to learn IPR status of the target and analyze finally the financial/economical realism of the new development program. Next, students design a program for the drug discovery phase by analyzing methodological possibilities to find a lead compound and to optimize it for further development. After this students design entire non-clinical development program for the drug candidate during which they plan all studies to demonstrate mechanisms of action of the drug molecule, ADME-properties, efficacy in animal disease models and toxicological and safety experiments that are required to demonstrate safety of the candidate molecule. Finally, students design clinical development program for the study drug beginning from the planning first-to-man dosing studies in healthy volunteers to phase 2 and 3 clinical studies in patients. Communication and presentation skills will be practiced during the entire course of the study module as a built-in study component. Contents The study module is based on project and team work. Team will be given the name of a target protein and team starts working to produce both written project reports and give oral presentations of the following topics: a) evaluation of the target as a possible drug development target and outline of the the project plan and time table, b) drug discovery phase of the project, c) non-clinical phase of the project and d) clinical phase of the project Students also keep notes of the project meetings. Thus, during this study module students write 4 project reports and keep 4 oral presentations. As a part of the project, students will have lectures of general outlines of project work. They will also get advice on presentation skills by an expert. Language of the project reports will be checked by a native English teacher who gives feedback to students. Teaching methods Project work, group work, lectures Teaching language English Modes of study Available for degree programme students. Evaluation and evaluation criteria Numerical grade (0-5). Evaluation criteria are based on the outcome of the project work. For this, both written project reports as well as oral presentations will be evaluated. Scientific quality and reasoning of approaches used during different phases of the project, innovative ideas, knowledge of regulatory requirements of drug development processes and linguistic performance will be taken into account in the evaluation. Recommended year of study First year of Master’s studies, spring semester. Study materials Given material on the project and team work. Students are encouraged to seek information of relevant regulatory guidelines from drug discovery and development available on web pages of EMA, FDA and ICH, and utilize IPR (patent) databases, PuBMed, Clinical Trials Register and other information sources. 29 DRUG0013 Bioentrepreneurship: Seizing Opportunities (5 ECTS) Learning outcomes The course gives an understanding of the process of identifying, exploring, and developing an entrepreneurial opportunity that best leverages your experience, interests, passions and networks. Content The course content relies on opportunity recognition, a theory based on entrepreneurship. The course explores concretely the entrepreneurial process from the beginning of a pre-idea phase to the actual business creation through opportunity recognition, opportunity assessment, and opportunity exploitation. The opportunity recognition is considered the first critical step in starting a new business. During that phase, an opportunity is first recognized and then evaluated. In the process a decision to move forward, that is to exploit the opportunity, is a result of the opportunity recognition process. According to the theory of opportunity recognition, people use a specific cognitive process to recognize the potential in a new business opportunity. The idea is based on considering past experiences, risks and market trends to recognize the potential of a new business opportunity. The students will simulate this process through a practical set of exercises, which leads them from the idea generation through idea evaluation into creating a business model in the field of bio sciences. Teaching methods Lectures and seminars (24 h), exercises, group work, written reflection paper Teaching language English Evaluation and evaluation criteria Numerical grade (0-5). No examination. Recommended year of study First year Master’s studies, spring semester. Previous studies Students might consider taking Bioentrepreneurship I and II (FALL1501, FALL1502) Study materials Literature is to be specified in the beginning of the course by the responsible teacher 30 Master’s thesis (55 ECTS) Learning outcomes: The main goal of the study module is that students write a research plan, conduct actual research at laboratory, analyze results obtained and demonstrate their ability to interpret results and write a report in a form of scientific article. Furthermore, as a built-in module of practising scientific communication, students present their results in seminars and write a press release in order to simulate collaboration of between the academia and media. Contents: The study module is divided to a) Master’s thesis research project work (35 ECTS) that includes practical laboratory work and participation in proseminars and pro gradu-seminars, and b) Master’s thesis research report (20 ECTS). Detailed guidelines and time table are given separately. Teaching methods: Practicing scientific English by writing research plan, keeping presentations and writing Master’ s thesis. Laboratory work. Teaching language English Modes of study Available for degree programme students. Evaluation and evaluation criteria Numerical grade (1= sufficient; 2=satisfactory;3=good; 4=very good; 5 =excellent). Evaluation criteria are based on 11 different categories (see separate guidelines). Recommended year of study Second year of Master’s studies. 31 Elective studies Recommended studies for those whose degree is not awarded in Finland: BIMA2105 Biomedical Ethics 1 ECTS Persons in charge Veikko Launis Objectives Content: Principles of Biomedical Ethics, Risk and Uncertainty in Modern Bioethics, Natural and Unnatural in Biomedicine, Case studies Modes of study Lectures 8h (4x 2h), mini essays Evaluation Pass/fail Previous studies Recommended year of study 1. Year Study materials Lectures KIFF0003 Finnish for Foreigners, Intensive Beginners Course 5 ECTS Offered by the Language Centre. See https://nettiopsu.utu.fi/opas/laitos.htm?opsId=214&uiLang=en&lang=en&lvv=2014 for course description. 32