- Te Aho o Te Kura Pounamu

te mĀtauranga pŪtaiao the sciences
SC1041 heat on the move ncea level 1
2011 / 1
physics and the physical sciences ncea level 1
Expected time to complete work
This work will take you about 12 hours to complete.
You will work towards the following standards:
Achievement Standard 90939 (Version 1) Physics 1.5
Demonstrate understanding of aspects of heat
Level 1, External
4 credits or
Achievement Standard 90943 (Version 1) Science 1.4
Investigate implications of heat in everyday life
Level 1, Internal
4 credits
In this booklet you will focus on these learning outcomes:
• describing the difference between heat and temperature
• describing the particle arrangement in states of matter
• explaining the transfer of heat by conduction, convection and radiation
• explaining the applications of heat transfer ideas in real-life situations.
This is the first booklet working towards both standards.
You will continue to work towards AS90943 in:
• Dressing for extremes (SC1042).
You will continue to work towards AS90939 in:
• Heat and particles (SC1211)
• Heat in action (SC1212).
Copyright © 2011 Board of Trustees of Te Aho o Te Kura Pounamu, Private Bag 39992, Wellington Mail Centre, Lower Hutt 5045,
New Zealand. All rights reserved. No part of this publication may be reproduced or transmitted in any form or by any means without the written permission of Te Aho o Te Kura Pounamu.
© te aho o te kura pounamu
contents
1 Nature of energy
2 Internal energy
3 Measuring temperature
4 Matter
5 Conduction
6 Insulators
7 Convection
8 Radiation
9 Answer guide
© te aho o te kura pounamu SC1041 1
how to do the work
When you see:
1A Complete the activity.
Check your answers.
Contact your teacher.
Use a computer.
You will need:
• the thermometer (0 – 100º) provided in this booklet
• a pen and a pencil
• a rubber band
• three water glasses, ice water, tap water, hot water, food colouring, three plastic bags, twist ties, a cup of sugar
• cornstarch, mixing bowl, square cake pan
• spoon, butter, metal knife
• a wooden spoon, a plastic spoon, a metal spoon
• a large container
• a black object, a white object
• a toaster, a metal tray, a wooden chopping board, a coffee mug
• a sheet of paper, cardboard, a tea towel, polypropylene jacket, aluminium foil
• a cooking pot, handful of macaroni, popcorn kernels, a coloured ice cube, a small glass container.
• internet access and access to the Topic website (recommended for all lessons).
© te aho o te kura pounamu 2 SC1041
1 nature of energy
learning intentions
In this lesson you will learn to:
• describe the meaning of the terms ‘energy’ and ‘work’
• describe different forms of energy
• outline transformation of energy in a given situation.
what is energy?
Energy is all around you. Energy causes things to happen. Energy helps us to do things. It gives us light. It warms our bodies and homes. We use energy for everything we do. We use it to bake cakes and keep ice cream cold. It runs our TVs, cell phones and cars. We need energy to grow and live. Even thinking requires energy. Without energy, nothing would ever change, nothing would ever happen.
So what is energy?
Energy is the ability to do work.
Energy and work occupy an important part in our ordinary lives and are among the most important topics in physics. Work in physics has a different meaning from the word ‘work’ that we normally use.
In physics, work is done when a force (push or pull) moves an object in a certain direction. What does it mean? Let us look at the following situation.
Here, the woman is being pulled by a dog. The force applied by the dog on the woman makes her move forward. So the dog is using its energy to pull the woman. In physics we say ‘the dog is doing the work on the woman’. Here, energy is transferred from the dog to the woman and that is what makes her move forward.
We can see that energy and work are inseparable. In physics, energy and work describe the same thing. Whenever energy is spent, work is always done, or whenever work is done, energy is spent.
We can define energy and work in this way:
Energy is the ability to do work. Work is the transfer of energy.
© te aho o te kura pounamu SC1041 3
nature of energy
forms of energy
Energy exists in many different forms. The chart below shows some of the common forms of energy.
Energy can be stored for a long time – like electrical energy in a battery or chemical energy in a tank of diesel fuel. The stored energy is known as potential energy and it can be changed into another form of energy.
Water in an overhead tank has gravitational potential energy because when the tap is turned on the force of gravity makes the water run.
Light energy Kinetic energy
Heat energy Forms of energy Sound energy
Chemical energy Electrical energy
Kinetic energy is the energy of moving objects or mass. Wind energy is an example of kinetic energy. The gas molecules in the air are moving, giving them kinetic energy.
The flowing water in a river has kinetic energy.
A running person has kinetic energy.
The amount of kinetic energy of an object depends on how fast it is moving. The faster an object moves, the greater its kinetic energy.
For example, if the speed of an object doubles then its kinetic energy increases by four times.
4 SC1041 © te aho o te kura pounamu
types of energy
Although there are many forms of energy there are also specific types of energy. For example, there are many types of potential energy.
Some of the different types of potential energy are:
• chemical potential energy – such as in petrol or in a battery
• elastic potential energy – such as in a stretched rubber band or
a compressed spring
• gravitational potential energy – such as in the overhead
Mechanical energy can be either kinetic energy or gravitational potential energy.
1A quick quiz
1. Name the energy stored in a rubber mattress when a person is
lying on it.
nature of energy
2. Name the energy stored in a bar of chocolate.
Check your answers.
© te aho o te kura pounamu SC1041 5
nature of energy
energy transformation
Energy can be transformed from one form to another. Things happen when energy is transformed.
For example, when you listen to an MP3 player, chemical energy from the battery is changed into electrical energy and then into sound energy. Sound waves make your ear drums vibrate, which is kinetic energy. This is an example of energy transformation that makes things happen.
When your body feels cold, heat is flowing too out of it fast.
When you bake a cake, heat is flowing from the oven heating elements into the cake. These are examples of energy changing location.
When you use an electric beater, electrical energy is changed into the kinetic energy of the prongs, heat energy causes the beater to get warm and sound energy causes it to make noise.
The useful part is the kinetic energy of the prongs.
1B quick quiz
1. a. Name the type of potential energy in a matchstick.
b. Write down the energy transformations that take place when the
matchstick is lit.
2.
What kind of energy transformations take place when a
speaker plays music?
Check your answers .
6 SC1041 © te aho o te kura pounamu
energy does not disappear
Energy is never lost and it never disappears. There is the same amount of energy today as there was when the world began. We change it into different forms of energy. When we burn firewood, we change its energy into heat and light. When we drive a car, we change the energy in the fuel into heat, sound and kinetic energy. This is called the energy conservation principle .
The principle of conservation of energy states that:
Energy cannot be created or destroyed; it can only be changed from one form to another.
There will always be the same amount of energy in the world. But more and more of it will be changed into heat. Most of that heat will go into the air. Energy will therefore still be there, but it will be hard to use.
key points
• Energy is the ability to do work. Work is the transfer of energy.
When we do work we always transfer energy.
• Energy exists in many different forms. Sound energy, heat energy,
kinetic energy and potential energy are some examples of
different forms of energy.
• There are also specific types of energy. Examples of different
types of potential energy are chemical potential energy, elastic
potential energy and gravitational potential energy.
• For anything to happen there has to be a transfer or change or
conversion of energy – such as from chemical to heat or potential
• We can never create or destroy energy; we can only change the
existing energy from one form into another. nature of energy
© te aho o te kura pounamu SC1041 7
nature of energy
1C check your understanding
1. A boy sits on the top of a sand dune. When he slides down
the dune the force of gravity pulls him down.
a. Name the form of energy he has when he is on top
of the dune.
b. Write down the energy changes taking place
in this situation.
2. Describe the energy changes taking place in a torch light
when it is turned on.
3. The photo shows a car being driven with its lights on.
a. Write down four forms of energy associated with this car.
b. Name the main form of energy used to power the car.
transformation of energy taking place in the car.
8 SC1041 © te aho o te kura pounamu
4. Amy throws a stone up in the air. It falls in a pond creating
ripples. Draw an energy flow chart showing the
transformation of energy taking place in this situation.
nature of energy
5. Select an appropriate situation to explain the meaning of the term
‘conservation of energy’.
6. When potatoes are cooked in a pot without a lid you could say that
energy is ‘lost’. Using ideas of physics explain why energy is never
really lost.
Check your answers.
© te aho o te kura pounamu SC1041 9
2 internal energy
learning intentions
In this lesson you will learn to:
• describe the meaning of the terms ‘ internal energy ’ and ‘ heat ’
• state the unit of heat energy.
introduction
In the last lesson you learned that energy can take on many forms. One important form of energy required for life on Earth is heat energy. Our main source of energy is the Sun. Light from the Sun is converted to heat and without heat energy there would be no life on Earth.
Many different types of energy can be converted into heat energy. Light, electrical, mechanical, chemical, nuclear, and sound energy can each cause a substance to heat up. Generally, if we put energy into an object, it heats up; if we take energy away from the object, it cools down. For example, when you are cold, you can jump up and down to get warmer; and when you sit down energy escapes from your body, so you feel cold.
2A hands-on activity: paper clip
What you need:
• a paper clip.
What you do:
1. Straighten the paper clip to a piece of wire.
2. Now bend the wire back and forth five times
and feel the bent part of it on your lips.
3. What do you feel? Explain why.
Check your answers.
10 SC1041 © te aho o te kura pounamu
You will have noticed that people rub their hands together on a cold day. Do you know why they do it? When hands are rubbed together, friction between the hands causes heat. Here mechanical energy is converted to heat energy.
Some of the chemical potential energy in food is converted to heat energy by your body.
When you exercise you convert chemical potential energy in food to mechanical energy and to heat energy.
Chemical energy
Heat energy is the most common form of energy on earth. Without heat energy we cannot survive – life is virtually non-existent without heat energy.
what is heat energy?
To answer this question you need to know what is inside matter.
All matter is made of very small particles called ‘atoms’. These atoms are generally joined together in groups called ‘molecules’. The atoms or molecules in a substance are usually moving and colliding with each other. The speed they move at depends on the energy they have. internal energy
Heat energy
Mechanical energy
It may seem strange to think that the molecules in a table or chair are moving, but they are. A moving object always has kinetic energy.
By definition, heat is the kinetic energy of atoms or molecules that are vibrating or moving around.
If the molecules are moving around, it means they have energy. If you suddenly stop them from moving, then some of the energy has to go somewhere else.
Compared to a warm object, the molecules in a cold object have less molecular motion, so they have less kinetic energy and therefore less heat energy.
© te aho o te kura pounamu SC1041 11
internal energy
2B hands-on activity: rubber band trick
What you need:
• a thick rubber band
• a plastic bag (e.g. a Te Kura plastic envelope).
What you do:
1. Put a rubber band to your forehead and feel the
temperature. Do you feel hot or cold?
2. Keep holding the rubber band touching your forehead.
Now stretch it out quickly as far as you can. Do you feel
hot or cold?
3. Let the rubber band shrink back down quickly.
Do you feel hot or cold?
4. Repeat the same experiment using a 1 cm strip of plastic
bag. You will not be able to do the second part of the
experiment where you let the rubber band go. The plastic
bag will not shrink back once it is stretched out.
Write down what you felt and compare the result to your
answer to question 2.
Check your answers.
Why does the rubber band behave this way?
The rubber band and plastic bag are made of long molecules. When you pull them you increase their kinetic energy, so they feel hotter. The molecules in rubber can pull back – when they do this they lose kinetic energy, so they get colder. Scientists are working on using this effect to make plastics that keep themselves cool.
12 SC1041 © te aho o te kura pounamu
internal energy
internal energy and heat
The total heat energy of an object is often called the internal energy.
Internal energy is the energy that an object has because of the motion of its molecules. It is the combined kinetic and potential energy of an object.
Internal energy is the total amount of heat energy of all molecules in an object.
When a cup of hot chocolate is heated energy is added to it. The molecules in the liquid move faster, so the internal energy increases.
When it cools down, heat energy escapes, the internal energy decreases and the molecules slow down.
The total heat energy of a cup of hot chocolate is dependent on the amount of drink in the container. A small cup of hot chocolate has fewer molecules than a large cup. So it has a smaller amount of internal energy than the large cup.
We often use the word ‘heat’ in everyday conversation. In physics, heat has a different meaning.
A large cup of hot chocolate has a greater number of molecules and a greater amount of internal heat energy.
Heat is the flow of energy from one point to another because of a temperature difference.
Heat Heat
Heat flows from the hot candle flame into the room.
Heat energy always flows from a hot object to a cold object.
For example, the heat flows out from a fireplace into the room.
Scientists sometimes use the word ‘thermal’ to mean ‘heat’. It does not matter whether you say ‘heat energy’ or ‘thermal energy’, they both mean the same thing.
A small cup of hot chocolate has fewer molecules and less internal heat energy.
© te aho o te kura pounamu SC1041 13
internal energy
How can the amount of heat energy be measured?
The internationally agreed unit (SI unit) of heat energy is the joule (J). (Joules, the plural, sounds exactly like the word ‘jewels’, as in rubies and emeralds.)
Since energy and work are the same thing, we can use the concept of work to measure energy.
One joule is the amount of energy needed to lift a 500 g block of butter to a height of 20 cm. When you lift a 1 kg block of cheese to a height of 10 cm you would use one joule of energy.
A 100 watt light bulb uses 100 joules of energy each second.
The term ‘joule’ is named after an
English scientist James Prescott Joule, who lived from 1818 to 1889.
He discovered that heat is a type of energy.
One joule is a very small amount of energy. A small activity requires thousands of joules of energy. Usually we measure energy in kilojoules (kJ).
1000 joules = 1 kilojoule
A glass of orange juice contains 350 kJ (350 000 J) of energy.
A piece of buttered toast contains about 315 kJ (315 000 J) of energy. With that energy you could:
• jog for 6 minutes
• bicycle for 10 minutes
• walk briskly for 15 minutes
• sleep for 1½ hours.
2C quick quiz
How many joules of energy are used up by a person who walks briskly for an hour? Use the information above to do a calculation.
Check your answers.
14 SC1041 © te aho o te kura pounamu
key points
• Internal energy is the energy an object has because of the
movement of its atoms and molecules, which are continuously
jiggling and moving around.
• When we add energy to an object, its atoms and molecules move
faster, increasing its energy of motion or internal energy.
Even objects that are very cold have some internal energy because
their atoms are still moving.
• Heat is the term used to describe the transfer of energy from
a hot object to a cold object.
• We use the unit joules (J) to measure heat energy.
2D check your understanding
1. A glass of water is left standing in bright, hot sunshine.
What happens to the water particles?
2. What is internal energy? What units are used to measure it?
internal energy
3. Compare the total heat energy contained in a hot water tank at
home with the total heat energy contained in a massive iceberg.
Which would be the greater? Explain your answer.
© te aho o te kura pounamu SC1041 15
internal energy
4. In the United States, the energy content of food is usually measured
in calories. For example, the energy value of 10 g of jam is equal to
27 calories. One calorie equals 4.186 joules. Calculate the amount
of energy in joules in 10 g of jam.
5. Does the heat energy contained in a hot pizza depend on the mass of
the pizza? Explain your answer.
6. A medium-sized chocolate Easter egg (39 g) contains 790 kJ of
energy. A person uses 20 kJ of energy per minute during brisk
walking. How many minutes of brisk walking are required to use up
the energy in the chocolate Easter egg?
Check your answers.
16 SC1041 © te aho o te kura pounamu
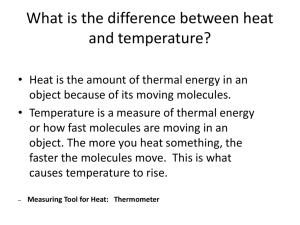
3 measuring temperature
learning intentions
In this lesson you will learn to:
• describe the meaning of the term ‘temperature’
• state the difference between temperature and heat energy
• state the units of measurement of heat and temperature.
introduction
In the previous lesson we discussed the idea that the internal energy or heat energy of an object is dependent on the total kinetic energy of all the particles in it.
Knowing the difference between heat energy and temperature is important. In this lesson we will define temperature and reach an understanding of how heat energy and temperature are related but are not the same thing.
a wrong idea
Heat energy (internal energy) and temperature are often thought to be the same – they are not.
Perhaps the reason the two are usually and incorrectly thought to be the same is because experience leads us to notice that when you heat something up, for example, putting a pot of water on the stove, the temperature of water goes up. More heat, more temperature – they must be the same, right? No, this is not true.
Is internal energy (heat energy) the same as the temperature?
To begin, the water in both the tank and the jug are at same temperature and both are heated with the heating elements of the same power.
The electric jug contains 2 L of water and is heated with a
2 kW heating element.
You will know from experience that the jug of water will heat up more quickly than the water in the hot water tank. But both have had exactly the same amount of heat energy put into them.
If thermometers were put into both of them, the jug would feel much hotter because the water particles would be moving much faster. The same heat energy put into the hot water tank has been shared between many more particles so they will have increased speed but not by nearly as much as the particles in the jug.
This shows that temperature and heat energy are two different things.
The hot water tank contains
200 L of water and is heated with a 2 kW heating element.
© te aho o te kura pounamu SC1041 17
measuring temperature
Does this mean that a cold lake contains more total heat energy than a pot of boiling water, even though the pot of water is very hot?
The answer is yes.
3A hands-on activity: hot or cold – human thermometer
Most of the time we can tell if something is hot or cold by touching it, but not always. Try out the following activity.
What you need:
• three glasses
• ice water, tap water and hot water.
What you do:
1. Fill one glass with hot water (not hot enough to hurt your hand),
one with ice water (take the ice out before you begin), and one with
tap water.
2. Put the index finger of one hand in the hot water and the index finger
of the other hand in the cold ice water. Keep them in the water for
about one minute.
3. Put the finger from the hot water into the glass of tap water.
What did you feel?
18 SC1041 © te aho o te kura pounamu
4. Put the finger from the ice water into the glass of tap water.
What did you feel? Did you feel hot or cold or both? measuring temperature
5. What does the result suggest about the way in which the skin
responds to temperature?
Check your answers.
© te aho o te kura pounamu SC1041 19
measuring temperature
3B hands-on activity: hot or cold – particle motion
What you need:
• three glasses
• some cold water, tap water, and hot water
• food colouring.
What you do:
1. Take three drinking glasses. In one glass add
cold water from the fridge, in the second glass
add same amount of water at room temperature
(straight from the tap), and in the third one
add very hot water.
Food colouring
2. Place all three glasses of water next to each
other on a bench. Wait until the water in all
three glasses appears still. Then, as quickly
as possible, add two drops of the food
colouring into each glass. Add the food
colouring with minimal splashing so that it
enters the water gently. For the best effect,
the colouring should be added to the cold
Cold
glass first, then the room temperature glass and last to the hot water
glass. Now watch and see how fast the food colouring spreads in
each of the glasses.
Medium
Write down what happened and explain in your own words why
it happened.
Hot
Check your answers.
20 SC1041 © te aho o te kura pounamu
measuring temperature
hot and cold
The difference between hot things and cold things is referred to as temperature. Temperature is a measure of how hot something is.
The temperature of an object depends on the kinetic energy of its molecules. The water molecules in each glass do not appear to be moving differently from each other. But they are.
Temperature is a measure of the average kinetic energy of the molecules of a substance.
The atoms and molecules in a substance do not always travel at the same speed. This means that there is a range of kinetic energy among the molecules. In a gas, for example, the molecules are travelling in random directions at a variety of speeds – some are fast and some are slow.
Sometimes these molecules collide with each other. When this happens the higher-speed molecule transfers some of its energy to the slower molecule, causing the slower molecule to speed up and the faster molecule to slow down.
For example, if hot water is added to cold water then some faster moving particles (hot) will collide with some slower particles (cold). The faster particles will be slowed down by the collisions and the slower particles will speed up. The average speed of the particles will be somewhere between the original fast and slow speeds. The final temperature of the mixture will be somewhere between the temperatures of the cold water and the hot water.
Since temperature is an average measurement, it does not depend on the number of particles in an object or the mass of the material. Temperature is not energy, but a measure of it. Heat is energy.
Temperature
=
Degrees of hotness
Heat energy
=
Amount of total energy in a substance
© te aho o te kura pounamu SC1041 21
measuring temperature
measuring temperature
Instruments for measuring temperature are called thermometers .
Temperature is a measure of how hot something is and is usually measured in degrees Celsius (ºC). Thermometers measure the average kinetic energy of the particles.
There are different kinds of thermometers. If a liquid is enclosed in a glass bulb with a thin tube attached, then the liquid moves up the tube when it is heated. We use the rise in level of the liquid as a measure of temperature.
Some thermometers use mercury as the liquid because it allows heat to move through it quickly, so you do not have to wait long for the liquid level to settle. Others use coloured alcohol as the liquid, because it is safer than mercury. We also use digital thermometers to measure temperature.
110ºC
100ºC
90ºC
80ºC
70ºC
60ºC
50ºC
40ºC
30ºC
20ºC
10ºC
0º
The United States uses the Fahrenheit scale to measure temperatures.
In this scale, 32ºF is the freezing point of water and 212ºF is the boiling point.
Water boils at 100ºC
Normal human body temperature is 37ºC
Water freezes at 0ºC
The diagram shows a mercury thermometer that can measure temperatures from 0ºC to 110ºC.
Fahrenheit
Boiling
212º
200º 160º
Human body temp.
98.6º
120º 80º
Freezing
60º
32º
40º –40º
80º 60º 40º
37º
20º –20º –40º
100º
Celsius
0º
Scientists also use another scale for measuring temperature called the Kelvin scale. In this scale 273 K is the freezing point of water and 373 K is the boiling point of water.
Theoretically, at – 273ºC the molecules in a substance stop moving completely. This is called absolute zero . Temperature, in Kelvin, is directly proportional to the average kinetic energy of the molecules.
One degree in the Kelvin scale is the same as one degree in the
Celsius scale.
373 K
273 K
0 K
100ºC boiling water
0º melting ice
-273ºC absolute zero
22 SC1041 © te aho o te kura pounamu
measuring temperature
heat flow
Heat and temperature are closely linked but they are not the same.
We can tell that something is hot or cold by measuring its temperature.
The flow of heat is always from hot to cold. We call this a thermal gradient .
key points
• Temperature is a number that is related to the average kinetic
energy of the molecules of a substance.
• Heat is a measurement of the total energy in a substance.
That total energy is made up of not only of the kinetic energies
of the molecules of the substance, but total energy is also
made up of the potential energies of the molecules.
• Temperature is most commonly measured in degrees Celsius (ºC).
• Heat is measured in joules (J).
• Heat flows from areas of higher temperature to areas of lower
temperature.
Heat flows from areas of higher temperature to areas of lower temperature.
3C check your understanding
1. State which of the following containers have the greatest amount
of heat energy.
a. A large bucket of tap water
b. A mug of boiling hot water
Explain your answer.
Around 1743, Anders Celsius
(1701–1744) invented the
Celsius scale. He determined the freezing temperature for water to be 0 degrees and the boiling temperature
100 degrees. The Celsius scale is known as a Universal System
Unit. It is used throughout science and in most countries.
© te aho o te kura pounamu SC1041 23
measuring temperature
2. What is ‘degree of hotness’? Name its unit of measurement.
3. Decide whether the following statement is true or false: ‘Degree of
hotness depends on the speed of the molecules.’ Give a reason for
your answer.
4. Decide whether the following statement is true or false:
‘Quantity of hotness (heat) depends on the speed and mass of the
molecules.’ Give a reason for your answer.
24 SC1041 © te aho o te kura pounamu
5. Water is being heated on a stove. Describe what happens
to the water molecules while it is being heated.
measuring temperature
Check your answers.
© te aho o te kura pounamu SC1041 25
4 matter
learning intentions
In this lesson you will learn to:
• describe the states of matter using illustrations and descriptions
• describe and compare the arrangement of molecules in a solid,
liquid and gas
• relate the states of matter to the quantity of heat. introduction
Look around you. All the stuff around you – your chair, the water you drink, the air you breathe – is matter.
Matter is the word we use to describe all the material in the universe.
It is anything that has mass and occupies space. The universe is made up of matter. Scientists study matter to find out more about the universe.
matter and energy
Matter can exist in different states or phases. What are the states of matter (or phases of matter)?
The state of matter is always related to how much heat energy is contained within the molecules of matter. The more heat energy that is added, the more the molecules move around and the harder it is for them to stay close together. So we classify matter based upon:
• particle arrangement
• energy of particles
• distance between particles.
26 SC1041 © te aho o te kura pounamu
4A hands-on activity: feel the difference
What you need:
• a cup of sugar
• water
• three small plastic bags and something to tie
the bags tight, for example, twist ties.
What you do:
1. In one plastic bag pour in the cup of sugar. Shake the bag so that the
sugar settles in it. Squeeze all the air out and tie it tightly so that no
air is left in the bag.
2. In the second plastic bag pour in a cup of water. Squeeze all the air
out and tie it tightly so that no air is left in the bag.
3. Blow some air in the third plastic bag and tie it tightly.
4. Squeeze each bag between your fingers and feel the difference.
Write down what you felt.
Check your answers.
matter
© te aho o te kura pounamu SC1041 27
matter
states of matter
There are three common states of matter:
• solid
• liquid
• gas.
There is a fourth state of matter called ‘plasma’. We will not study this state in this topic.
properties of solids
Sugar is an example of a solid. Sugar feels hard and the crystals of the sugar have a definite shape.
• Particles in solids are tightly packed and vibrating about a fixed position.
• Solids have a definite shape and a definite volume because the particles
are locked into place and cannot move or slide past one another.
• Solids are not easily compressible because there is little free space
28 SC1041 © te aho o te kura pounamu
properties of liquids
Water is a liquid. When squeezed, it feels ‘soft’, it wobbles and changes its shape. matter
• Particles in liquids are loosely packed and are far enough apart
to slide over one another; they are in motion.
• Liquids have no fixed shape and they always take the shape of the
container. properties of gases
Air is a gas. When the bag with air is squeezed, it feels ‘softer’ than sugar or water; it is very wobbly and changes its shape easily.
• Particles in gases are very far apart.
• Gases have no particular shape or volume because the particles
are always in motion.
• There is very little or no force holding the particles together so
they move freely.
• Gases flow very easily because the particles randomly move past
© te aho o te kura pounamu SC1041 29
matter
4B hands-on activity: silly state
This is an optional activity.
What you need:
• ¼ box of cornstarch flour and some extra flour
• large mixing bowl
• square cake pan, or a metal oven tray
• some water
• spoon to stir
• food colouring.
What you do:
1. Pour approximately ¼ of the box of cornstarch into the mixing bowl
and slowly add about ½ cup of water. Add a few drops of the food
colouring. Stir and mix them using the spoon or your hand.
2. Continue adding cornstarch and water in small amounts until you
get a mixture that has the consistency of honey. As a general rule of
thumb, you’re looking for a mixture of roughly 10 parts cornstarch to
1 part water. Notice that the mixture gets thicker as you add more
cornstarch flour.
3. Sink your hand into the mixture and notice its unusual consistency.
Compare what it feels like to move your hand around slowly and then
very quickly.
4. Hit it with a spoon or punch it with you hand. Sink your entire hand
into the goo and try to grab the fluid and pull it up.
5. Drop a plastic toy animal into the cornstarch mixture and then try to
get it out.
30 SC1041 © te aho o te kura pounamu
6. Pour the mixture onto the oven metal tray or cake pan. Notice its
unusual consistency when you are pouring it onto the pan.
Stir it around with your finger, first slowly and then as fast as you
can. Skim your finger across the top of the glop. What do you notice?
Write down how you felt the mixture. Is it a solid or liquid? matter
Check your answers.
Why is it so gooey?
The cornstarch and water mixture acts like a solid sometimes and a liquid at other times. This mixture is an example of a suspension – a mixture of two substances, one of which is finely divided and dispersed in the other. In this case it’s a solid dispersed in a liquid.
When you punch the cornstarch mixture, you force the long starch molecules closer together. The impact of this force traps the water between the starch chains to form a semi-solid structure. When the pressure is released, the cornstarch flows again.
What does this gooey mixture have in common with quicksand?
Quicksand is nothing more than a soupy mixture of sand and water, where the sand is literally floating on water. Like the mixture, quicksand is actually a substance that behaves like a solid and a liquid at the same time. Quicksand is just solid ground that has been liquefied by too much water, and the term ‘quick’ refers to how easily the sand shifts when in this solid-liquid state.
© te aho o te kura pounamu SC1041 31
matter key points
• Three main states of matter are solid, liquid, and gas.
• Particles of solids are tightly packed, vibrating about a fixed
position. Solids have a definite shape and a definite volume.
• Particles of liquids are loosely packed, but are far enough apart
to slide over one another. Liquids have an indefinite shape and
a definite volume.
• Particles of gases are very far apart and move freely. Gases have
an indefinite shape and an indefinite volume.
4C check your understanding
1. Draw diagrams to show how particles are arranged in each of the
following items:
• a piece of metal
• a soft drink
• the gas released when a fizzy drink bottle is opened.
2. Complete the following table by filling in the correct answer.
States of matter What the molecules are doing
Liquid
Molecules are free from each other and rapidly moving.
Solid
32 SC1041 © te aho o te kura pounamu
3. Explain, in terms of their particle arrangement:
a. Why solids have a definite shape but liquids flow. matter
b. Why solids and liquids have a fixed size but gases will fill whatever
container they occupy.
Check your answers.
© te aho o te kura pounamu SC1041 33
5 conduction
learning intentions
In this lesson you will learn to:
• explain the process of conduction
• explain why some materials are better conductors than others
• describe the term ‘insulator’.
introduction
You may have noticed many times that on a cold day holding a cup containing a hot drink warms your hands. Why do your hands get warm?
Heat must be flowing between the drink and your hands because they are at different temperatures and touching one another.
Stir a saucepan of soup with a metal spoon and you will soon notice that heat travels rapidly along the spoon from the hot soup into your fingers.
Walk on a stone floor in your bare feet and it feels cold because heat flows out of your body into the floor.
If a cup of hot chocolate and a bowl of ice cream were left on the table in this room what would happen to them?
The cup of chocolate would cool until it reached room temperature.
The ice cream would melt and then warm to room temperature.
Our experience shows that heat always moves from a warmer place to a cooler place. Hot objects in a cooler room will cool to room temperature. Cold objects in a warmer room will heat up to room temperature.
© te aho o te kura pounamu 34 SC1041
5A hands-on activity: melting butter
What you need:
• a piece of butter
• a glass, a metal knife.
What you do:
1. Place the handle of the knife on the glass
and balance as shown in the photo.
2. Take five rice-grain sized pieces of butter.
3. Place the first piece 3 cm from the edge of the knife and the rest
about ½ cm apart on the knife.
4. Now place a lighted candle at the end of the knife.
5. Time how long it takes for each of the bits to show signs of melting.
a. Write down what you noticed.
b. Give an explanation for your answer.
Check your answers.
conduction
© te aho o te kura pounamu SC1041 35
conduction
what is conduction?
Conduction is one of the processes of heat transfer in matter. It takes place when two objects at different temperatures are in contact with each other. Heat flows from the warmer to the cooler object until they are both at the same temperature.
When you heat a metal strip at one end, the heat travels to the other end. As you heat the metal, the particles vibrate. These vibrations make the adjacent particles vibrate, and so on. The vibrations are passed along the metal and so is the heat. We call this conduction .
• Conduction is the flow of heat through matter from places of higher
temperature to places of lower temperature without the actual
movement of particles of the matter as a whole.
what causes conduction?
Conduction is the movement of heat through a substance by the collision of particles. At the place where heat is supplied, the particles gain kinetic energy and start vibrating faster. These particles collide with the slower vibrating particles at the cooler end and give up some of their energy to them. The slower particles gain more heat energy and collide with other particles adjacent to them.
This process continues until heat energy from the warmer object spreads throughout the cooler object.
do all materials conduct heat?
Yes, all materials conduct heat but some substances conduct heat more easily than others. Solids are better conductors than liquids; and liquids are better conductors than gases. Metals are very good conductors of heat, while air is a very poor conductor of heat.
5B hands-on activity: which one?
What you need:
• three similar, almost equal-sized spoons:
a wooden spoon (or stirring stick),
a plastic spoon, a metal spoon
• a large mug
• some hot water
• butter (or margarine).
Heat
Heat source
36 SC1041 © te aho o te kura pounamu
conduction
What you do:
1. Press a very small piece of cold butter onto the handle of each of the
three spoons.
2. Fill a large measuring cup or large mug ¾ full with hot water.
3. Place the three spoons in the water so that the handles of the
spoons come out of the top of the mug or cup.
4. Watch what happens.
a. In what order did the pieces of butter fall from the spoons?
b. Explain why.
Check your answers.
good and poor conductivity
A wooden rod and a copper tube are placed together end to end and paper is then wrapped around the two materials. A flame is gently passed back and forth over the paper. What do you think will happen?
Copper
The paper chars only where it is in contact with the wood. The paper touching the copper is unmarked. Why?
The reason is that the copper is such a good conductor that it can transfer the heat away before the paper begins to burn.
Paper
A video showing this is linked to the Topic webpage.
water is a poor conductor of heat
In this activity, a large test tube is filled with water.
An ice cube is put in the bottom of the tube by attaching a weight to it. (Ice has to be weighed down because it floats.)
The top of the tube is heated, the water boils and the bottom of the tube is cold and ice does not melt. Why?
Because water particles are in constant motion, it is a poor conductor of heat. It will take a few minutes before enough heat is conducted down to melt the ice.
A video showing this is linked to the Topic webpage.
© te aho o te kura pounamu
Wood water weight ice
SC1041 37
conduction
why are metals good conductors of heat?
Metals are particularly good conductors of heat because atoms in metals are very closely packed so the vibrations are passed on very quickly. They also contain large numbers of electrons that are free to move around. These are called ‘free electrons’. As the metal is heated, the free electrons closest to the heat source are heated. This makes them move faster and they travel through the metal, colliding with both atoms and other electrons, thus the heat is passed quickly through the metal.
5C
Atom
quick quiz
The normal temperature of human body is about 37.6ºC. On a warm day a bread knife is the same temperature as the room, which is about
20ºC. Why does the bread knife feel cold when you touch it?
Electron
Check your answer.
38 SC1041 © te aho o te kura pounamu
conduction
5D
use of good conductors
The ability to conduct heat is called ‘thermal conductivity’, which is measured in watts per metre per degree (W m –1 ºC –1 ). The table shows the thermal conductivities of some common materials.
Metal Conductivity a
Copper that excellent
Aluminium 201 .00
heat
Brass 110 .00
bases transfer
heat energy quickly and evenly to
Gold
Iron
296 .00
80 .00
pan
Steel 63 .00
the
Wood 0.15
Silver 419 .00
Glass used heat
Cork
1 .00
0.05
hot
Good conductors of heat are used when we want heat to be transferred quickly.
You will notice that a lot of different items in the kitchen are made of metals.
They are utensils that we want heat energy to transfer through easily, such as pots and pans and baking trays.
Aluminum and steel are the other two metals used to make kitchen utensils. quick quiz
1. From the table, list the two best and two worst conductors of heat energy.
2. Explain why it not a good idea to use spoons made from silver
metal to drink hot soup.
Check your answers.
key points
• Conduction is the flow of heat through matter from places of higher
temperature to places of lower temperature without the actual
movement of the particles of matter as a whole.
• All metals are good conductors of heat energy.
• Non-metals are poor conductors of heat energy.
© te aho o te kura pounamu SC1041 39
conduction
5E check your understanding
1. Identify kitchen items made of plastic, metal and wood. Explain the
uses of these items based on heat conductivity.
2. Use your knowledge of particle arrangement in a solid to explain
how conduction of heat takes place in a solid.
3. Explain why water can never be a good conductor.
40 SC1041 © te aho o te kura pounamu
4. The drawing shows rods of the same length and diameter that are
made from different substances. One end is inserted into a tank of
hot water. At the other end of the rods small pebbles are stuck on
with wax.
Pebble stuck on with wax
Tank of hot water
Equal length rods of different materials
a. In what order would the pebbles fall off if the rods were made of
glass, iron, wood, and copper? conduction
b. Explain why cork is used to make table mats on which hot dishes
Check your answers.
© te aho o te kura pounamu SC1041 41
6 insulators
learning intentions
In this lesson you will learn to:
• explain the meaning of the term ‘heat insulator’
• explain why some materials are better insulators than others
• describe the use of insulators.
6A hands-on activity: warm or cold
What you need:
• a metal oven tray and a wooden chopping wood.
What you do:
Take the metal oven tray and a wooden chopping board. Place your right hand on the wooden chopping board and your left hand on the metal tray.
What do you feel? Explain why.
The answer is that metal is a good conductor of heat; it absorbs and therefore removes heat from your hand, so you feel cold. Wood is a poor conductor of heat and is a reasonably good insulator. Wood conducts only a minute amount of heat away from your hand and it remains almost at body temperature. So, comparatively, the hand on the wooden board feels warmer than the hand on the metal tray.
Check your answer.
42 SC1041 © te aho o te kura pounamu
insulators
6B thermal insulators
A thermal insulator is a material that prevents heat from moving from one place to another.
When we do not want materials to transfer heat, such as saucepan handles and chilly bins, we use materials that are poor conductors of heat. Materials that conduct heat very poorly are called thermal insulators . This means that the heat takes a long time to pass from the hot side to the cold side of the thermal insulator.
hands-on activity: keeping it in
What sorts of materials make good insulators?
What you need:
• a coffee mug, some very hot water
jacket, alumimium foil.
What you do:
1. Wrap a piece of paper around a mug and fill the cup with
hot water. Wrap your hand on the outside of the paper,
hold it there for about a minute and see how hot it feels.
A cooking pot has a metal body and a plastic handle. What is the
• a sheet of paper, some cardboard, tea towel, polypropylene heat the pot quickly on a stovetop because metal is a good conductor of heat. You are able to pick the pot up without an oven glove because the plastic handle is a poor conductor of heat. Using a combination of materials makes the kitchen tools easier to use.
The hotter it feels on the outside, the more heat is escaping
from the inside, and the poorer the insulator is.
2. Try it with other materials. Make sure that you fill the mug with
fresh hot water for each material.
3. List the materials from hottest to the coldest.
Check your answers.
© te aho o te kura pounamu SC1041 43
insulators
good insulators of heat
Remember, in a solid, heat is transferred when particles collide.
The distance between the particles in an insulator is greater than that in a conductor. The electrons in an insulator are tightly bonded to the atoms, and there are no free electrons. The particles closer to the heat source gain kinetic energy and begin to vibrate. The greater space between the particles prevents collisions taking place and limits heat transfer.
Atom
Wood and plastics are poor conductors of heat, but they are reasonably good insulators. Wool and fiberglass and all gases including air are good insulators of heat.
Electron
44 SC1041 © te aho o te kura pounamu
thermal insulation
Thermal insulation is the method of preventing heat from escaping an area or from entering the area. We use thermal insulators to prevent heat loss. For example:
• On a cold day you keep yourself warm by wearing clothes that
keep the cold out and your body warmth in.
• If you have a hot drink, you may want to prevent it from becoming
cold by putting it in a thermos bottle.
• If you live in a hot country, you may want to prevent the
temperature inside the house from becoming the same as the
temperature outside by having the house well insulated.
Less dense materials are better insulators. The denser the material, the closer its atoms are together. That means the transfer of energy from one atom to the next is more effective. Thus, gases insulate better than liquids, which in turn insulate better than solids.
In most cases, insulation is used to prevent the conduction of heat.
A good insulator is an extremely poor conductor.
How well a material works as an insulator is normally given by its
R-value. The R-value of a material is its resistance to heat flow and is an indication of its ability to insulate. It is a standard way of telling how well a material will insulate. A higher R-value gives a greater insulation.
The table gives the R-value of some common materials.
Material
Hardwood (3 cm thick)
Brick (10 cm thick)
Concrete block (10 cm thick)
Fiberglass/Pink Batts (9 cm thick)
Flat glass (0.4 cm thick)
Air space (9 cm thick)
R-value
0.91
4.00
0.80
10.90
0.89
1.01
insulators
© te aho o te kura pounamu SC1041 45
insulators where is thermal insulation used?
1. Personal clothing
Oven gloves, wool blankets, padded jackets and woollen jerseys are some examples of items that are made from thermal insulators.
If you rip open an oven glove or a padded jacket you will find an artificial material called
Dacron. It is made out of fluffy fibres. This material traps very small pockets of air. Air is a poor conductor because air molecules are spaced further apart, so transfer of heat energy from one molecule to the next is reduced.
Imagine that you are climbing Mount Cook on a very cold day. You are wearing a very thick down jacket and pants to guard against the extreme cold. It’s not the down itself that keeps you warm. Rather, it has an enormous number of tiny air spaces trapped between the down filaments that stop conduction of heat from your body out to the cold atmosphere. If air were not such a good insulator, no amount of down would allow survival under such cold conditions.
If you stretch a woollen jersey then you will notice holes. How can a garment with minute holes all over it keep a person warm during a winter day?
Again, it has large number of tiny air spaces trapped between the woollen fibres that stop conduction of heat from you to the environment.
2. House insulation
A well-insulated house is a bit like dressing for the weather.
A wool sweater will keep you warm. A house needs to be wrapped with insulation to keep the cold out and the warm air in.
The two most common wall types are wood-frame and solid-brick.
The space between the outside and inside is lined with an insulating material such as Pink Batts.
The attic is often lined with insulating material and in really cold places there is also under-floor insulation.
Pink Batts in attic
Floor
Pink Batts Under-floor insulation
46 SC1041 © te aho o te kura pounamu
3. Hot water system
Insulating hot water tanks and pipes reduces heat loss. Normally the tank and the connecting hot water pipes are wrapped with insulating material. New hot water tanks come with built-in insulation. insulators
6C
4. Household items
Polystyrene disposable cups are an example of the use of insulation.
The outside of the cup is cool enough to pick up although the inside may contain hot tea or coffee. Chilly bins are insulated boxes to keep food and drinks at the desired temperature. Fridge walls and oven walls contain high R-value insulation materials to keep heat loss to a minimum. key points
• Thermal insulation is used to minimise the heat transfer in many
everyday situations. It is done by reducing conduction,
convection and/or radiation effects. The R-value is a standard of
measurement of this insulation.
• The thicker the insulation, the less heat is lost.
• Gases are poor conductors of heat, hence they are good insulators.
check your understanding
1. Explain, in terms of particle arrangement, why gases are better
insulators than liquids.
Polystyrene disposable cups are an example of the use of insulation.
© te aho o te kura pounamu SC1041 47
insulators
2. A wool sweater will keep you warm if the wind is not blowing and it
is not raining. Explain why a wool sweater will be ineffective if the
wind blows.
3. How do gloves protect your hands on a cold day?
4. Some shops wrap fish and chips in 2–3 layers of paper while others
pack them in a cardboard box. Which one do you think will keep the
food warmer for a longer period of time? Give reasons for your answer.
Check your answers.
48 SC1041 © te aho o te kura pounamu
7 convection
7A learning intentions
In this lesson you will learn to:
• identify convection as a heat transfer method
• use the particle model to explain the process of convection
• describe practical uses of convection at home.
introduction
This section looks at how heat is transferred by liquids and gases.
Remember, the particles in a liquid or a gas are in constant motion.
They are very loosely bonded to each other in liquids and are totally free to wander around in gases.
The cycling movement or the current created by convection is called the convection current .
A convection current takes place when a liquid or gas is heated; it expands, becomes less dense and floats upwards. hands-on activity: making popcorn
What you need:
• a cooking pot, a handful of popcorn kernels
• a microwave oven.
What you do:
1. Take a few popcorn kernels and drop them in
a glass of water. What happens?
Heat causes the particles to spread out, the water expands and they become less dense. The cooler dense water at the top sinks and the warmer, less dense water rises to the top. As the cooler water sinks it heats up and moves back to the top. The circular motion continues for as long as the water is being heated up.
Smaller volume, denser
2. Take a handful of popcorn kernels. Place them in a deep glass dish,
cover the dish and put it in a microwave oven. Turn the oven on high
for 3 minutes or until they pop. If you have no microwave oven you
could pop them on a stove cooker.
3. Take one popped corn, put it in a glass of water. What happens? Why?
Check your answers.
Larger volume, less dense
The popcorn activity demonstrates what less dense and dense mean.
You noticed that the corn kernel sinks to the bottom of the glass while the popped corn floats even though their mass has not changed. When the corn is popped it expands, the particles in the corn are further apart than the corn kernel. The same mass is spread out to a larger space so its volume increases. This makes them less dense.
© te aho o te kura pounamu SC1041 49
convection
7B
Cooler gas particles, denser Heated gas particles, less dense
The same thing happens when liquids and gases are heated. When they are heated particles gain kinetic energy and push apart, become less dense and rise up. Cool liquids and gases with slower moving particles are closer together and are therefore denser. Like the corn kernel they are heavier and sink to the bottom.
hands-on activity: frozen water
• a coloured ice cube or a dark-coloured ice
block (To make an ice cube, pour 2 drops
of food colouring into any pocket of an
ice tray, add water and freeze. Red or
green food colouring gives a better display.)
• long glass (a beer mug or water jug)
• water.
What you do:
1. Fill the jug with water. Drop the coloured ice cube in it. It will sink
first and then float to the top. If you are using an ice block, break a
piece off it and add to the water. Watch what happens. Write down
what happens and explain why.
Check your answer.
50 SC1041 © te aho o te kura pounamu
7C hands-on activity: warm water
If you're not sure what to do watch the video clips on the Topic webpage.
What you need:
• a small glass container such as an empty small
glass bottle or a small sherry glass
• a few drops of food colouring, a piece of
aluminium foil and some hot water
• a tall clear glass container such as a vase,
a measuring jug or a long beer jug that can hold
the smaller container and is at least twice as tall.
What you do:
1. Fill the small container with very hot water and add a few drops of
food colouring. Make sure it is full to the top. Cover the top of the
container with a piece of aluminum foil. Put a rubber band over the
foil around the rim to hold it tight. Put the container into the
large container.
2. Pour cold water into the large container until the water goes over the
top of the small container and nearly the top of the large container.
Wait until the water stops moving.
3. Use a stick or a skewer to poke two holes into the foil. If there is air
at the top of the coloured water, you need to tap it gently or wriggle
the skewer in the hole to release the air.
4. Remove the skewer and look through the large container from its side
to watch what happens.
5. Write down what happened and explain why.
convection
Check your answers.
© te aho o te kura pounamu SC1041 51
convection
natural convection currents
If you live near the sea, an ocean or a large lake you would experience land and sea breezes. These are caused by natural convection currents.
Water has a much greater heat capacity than land and, as a result, it warms up more slowly than the land and also cools down slower.
So during the day, there are temperature differences between a lake or the sea and the surrounding ground. This temperature difference causes convection currents.
During the day, as the sun shines on the land, it heats up faster than water. As the land radiates heat, the air over the land becomes warmer and less dense.
It rises and is replaced by cooler, denser air flowing in from over the water.
This causes an onshore wind, called a sea breeze.
Rising convection currents over the land are called ‘thermals’.
They are used by glider pilots to keep their planes in the air and by birds to stay aloft.
At night the water cools down more slowly than the land. As the water radiates heat, the air over the water becomes warmer and less dense. It rises and is replaced by cooler, denser air flowing in from the land.
This is called a land breeze.
52 SC1041 © te aho o te kura pounamu
heaters at home
The air in a room is gas. Gases transfer heat by convection. The heater is placed on the floor. The air above the heater heats up and becomes less dense. It then rises and is replaced by cooler, denser air at the floor level. The warmer air cools and descends. The circulation of air caused by the convection current heats the room.
Some heaters have electric fans to circulate the air, which increases the speed of the convection current.
refrigerator design
The air in a fridge cools the food by convection. Look inside a refrigerator.
You will see a cooling panel placed at the top back. key points
• Convection is the transfer of heat energy by the actual movement
of particles of the matter. The warmer particles transfer heat
energy by moving from one place to another.
• Convection occurs in liquids and gases only.
• Sea breezes and land breezes are caused by convection currents.
This older style fridge has the freezer compartment inside the main cabinet.
The freezer cools the air.
Cold air falls from the top and circulates the air.
convection
© te aho o te kura pounamu SC1041 53
convection
7D check your understanding
1. The diagram shows an electric jug filled with water. The heating
element of the jug is placed at the bottom.
a. Explain why the heating element is placed at the bottom.
b. Draw arrows to show the direction of the convection current in
the jug when the water is being heated.
c. Explain how the water reaches an even temperature.
Element
2. a. Explain what is meant by the term ‘convection current’.
b. Explain why heat transfer by convection takes place only in
liquids and gases but not in solids.
54 SC1041 © te aho o te kura pounamu
3. Some school gyms and community halls have wall heaters placed
near the ceiling. Explain why this is an inefficient way of space heating. convection
4. Hot air balloonists use a gas fire to float their balloons in the air.
Balloonists turn the flame on to make the balloon go up in the air.
To lower the balloon the flame is turned off.
Explain why the up and down motion of the balloon can be controlled
by turning the flame on and off.
Check your answers.
© te aho o te kura pounamu SC1041 55
8 radiation
learning intentions
In this lesson you will learn to:
• identify radiation as a heat transfer method using waves
• identify heat as infrared radiation
• describe how the colour and texture of surfaces affect radiation introduction
You might have experienced warming cold hands at a camp fire on a cold day. The heat energy travels sideways, so it is not travelling by convection. Heat transfer between the heat source and the heated object that does not rely upon particles is called thermal radiation .
8A
Sun Space Earth
The Sun warms the Earth by radiation.
We feel heat from the Sun on a sunny day. The energy is carried through the empty space between the Sun and the Earth where there are no particles to carry energy. Radiation does not need a solid, gas or liquid to transfer energy. The energy is carried by electromagnetic waves similar to light.
The men feel the heat from the campfire by radiation. Most of the heat energy from the fire goes straight up in a convection current.
Thermal radiation is also known as infrared (IR) radiation or heat radiation .
Thermal radiation is given off ( emitted ) by all surfaces. The colour of the surface affects how fast it it emitted. how does surface colour affect heat loss?
What you need:
• two empty washed tin cans the same size
• a candle and a way to light it
• a thermometer
• something to make lids for the cans.
(We used cardboard circles and put stones
• a kettle of hot water.
© te aho o te kura pounamu 56 SC1041
radiation
What you do:
1. Blacken one can by holding it a candle flame.
Try not to touch the black soot – it will come
off on your hands.
2. Put 250ml of hot water into both the black
can and the shiny can.
3. Measure their starting temperature and
record it in the table. Measure their
temperature every 10 minutes for 30 minutes.
Cans Starting temperature
Temperature after 10 minutes
Dull,
Black Can
Shiny Can
Temperature after 20 minutes
Temperature after 30 minutes
4. Which can cooled the fastest?
5. Which surface radiates most heat energy? How do you know the
difference in cooling is due to radiation?
© te aho o te kura pounamu SC1041 57
radiation
The amount of radiation from hot objects depends on its colour and texture. A good radiator or emitter of heat energy is the one that loses energy quickly. A dull black surface is a good radiator or emitter of heat energy.
A poor radiator or emitter of heat energy is the one that loses energy slowly. A shiny or white surface is a poor radiator .
The photo on the left was taken using a special camera that detects thermal radiation.
The photo on the right shows part of the same picture, taken using light.
The girl’s faces are brightest because they are warmest. Warm surfaces give off more radiation than cool surfaces.
Radiation also depends on the surface temperature area. Hotter surfaces emit faster. Larger surfaces emit faster.
absorbing radiation
radiation. they than they emit, they heat up.
Earth’s surface loses heat by radiation. It heats up during the day because it gets more radiation from the Sun than it loses.
At night, without radiation from the Sun, it cools down.
58 SC1041 © te aho o te kura pounamu
radiation
8B
which surfaces are the best absorbers of radiation?
What you need:
• two tin cans, use the silver and black ones from
the last activity
• water
• a thermometer
• something to make lids for the cans.
(We used cardboard circles and put stones on top)
• a sunny day (if the sun isn’t shining, you could wait
for better weather. If you have an electric fire you
could use that instead of the Sun).
What you do:
1. Place cans in a sunny place where they will all get the
same amount of sun. If you don’t have any sun,
use a radiant heater.
2. Put a cup of water in each can (250ml).
3. Cover each can with a saucer or piece of card.
4. Use the thermometer to measure the temperature
of the water in each can. Fill your results into the
table below.
5. Measure the temperature again half an hour later.
Fill your results into the table below.
6. If the cans stay in sunshine, try to measure their temperature after
another half hour. Fill your results into the table below.
Colour of the can
Starting temperature Temperature after ½ hour of sunshine
Temperature after 1 hour of sunshine
Black
Shiny
© te aho o te kura pounamu SC1041 59
radiation
Explain your results. Try to use the words temperature and radiation in a sentence.
Surfaces that are good emitters of radiation are also good absorbers. Dark, dull surfaces are the best emitters and absorbers.
Surfaces that are poor emitters of radiation are also poor absorbers.
Light, shiny surfaces are the worst emitters and absorbers.
where do we use radiation ideas?
space blankets
Have you ever been at the finish line of a marathon?
Did you wonder why, as the runners cross the line, they wrap themselves in what looks like thin blankets of aluminum foil?
These blankets help the athletes keep their body warm.
When they stop running, the combination of light clothes and wet skin means that they lose heat rapidly. There is a risk of hypothermia (dangerously low body temperature). To prevent hypothermia, the runners are wrapped in space blankets at
How does the space blanket reduce heat loss?
The silvered inside surface of the blanket reflects radiation back to the runner’s body. The silvered outside surface reduces heat loss by radiation. Wrapping the blanket around the body creates a barrier to reduce heat loss to the surroundings by convection. It also traps an insulating layer of air to reduce heat loss by conduction.
60 SC1041 © te aho o te kura pounamu
radiation
thermal imaging
The image of this car was made using a thermal camera. Driving the car has made some places hotter than others. The hottest parts are white – they are radiating lots of infra-red.
Car designers use images like this to find ways to reduce drag and improve efficiency as they test prototype cars.
key points
• Radiation is the process of heat transfer by electromagnetic waves.
• Radiant energy travels as infra-red waves, similar to light.
• All surfaces give off infra-red. The warmer the surface, the more
radiation it gives off.
• Radiation does not need particles to transfer heat energy. It is the
only way energy can be transferred across empty space.
• A dull black surface is a good absorber and good radiator of
• A bright shiny or white surface is a poor absorber and poor
radiator of radiant energy.
8C check your understanding
1. Two identical thermometers were placed in direct sunshine
on a summer day. Describe and explain the difference in their
temperature readings.
© te aho o te kura pounamu SC1041 61
radiation
2. Four containers are placed in front of a heater. They are all at an
equal distance from the heater and left for one hour. List them from
warmest to coolest.
3. a. Explain why the black road is hotter than
the white line.
b. Discuss how their temperatures will change
On a sunny day the black road is much hotter than the white lines. The girl on the left is burning her feet, but the girl on the right is fine.
4. Firefighters need to be able to get close to a burning building
without getting too hot. Should their clothes be black or shiny silver?
Explain your choice.
5. You want to keep your take-away food hot on the way home.
Would you use a black bag or a silver bag? Explain your choice.
Check your answers.
62 SC1041 © te aho o te kura pounamu
9 answer guide
1A
1B
1. nature of energy
1. Elastic potential energy
2. Chemical potential energy
1. a. Chemical potential energy
b. Chemical potential energy to heat energy
2. Electrical energy is changed to kinetic energy and sound energy.
1C 1. a. Gravitational potential energy
b. Gravitational potential energy to kinetic energy
2. Chemical energy from the battery to electrical energy to light energy
3. a. Four of: chemical potential energy, heat energy, kinetic energy and sound energy,
electrical energy, light energy
b. chemical potential energy
c.
Heat energy
Kinetic energy
Chemical potential energy
Sound energy
Electrical energy
4.
Kinetic energy of the water
Kinetic energy Gravitational potential energy
Sound energy of the ripples
5. You will have your own answer here. For example, a child on a swing has a maximum potential
energy at the top of the swing's path. As the swing reaches the lowest point in its path she and
the swing have maximum kinetic energy. Potential energy lost is equal to kinetic energy
gained and energy is conserved.
6. Heat energy has been transferred from the pot into the air. It is not lost, simply hard to use.
© te aho o te kura pounamu SC1041 63
answer guide
2A
2B
2C
2D
2. internal energy
The paper clip feels warm. This is because the kinetic energy created when the paper clip is bent is converted into heat energy.
1. Cold
2. Hot
3. Cool
4. The plastic felt cool on my forehead and warmer after stretching.
315 kJ of energy is used when walking for 15 minutes
4 × 315 kJ is used in 1 hour = 1260 kJ = 1 260 000 J
1. The water particles absorb heat energy from the sun and start to move faster.
2. Internal energy is the energy an object has because of the movement of its particles.
It is measured in joules (J).
3. The total heat energy of the iceberg is greater because it has a much greater number of
particles with a greater amount of heat energy than the home hot water tank.
4. 1 calorie = 4.186 J
10 g jam = 27 cal
= 27 × 4.186 J
= 113 J
5. Yes, because heat energy depends on the number of particles which depends on mass.
6. Number of kilojoules of energy in an Easter egg = 790 kJ
Number of minutes brisk walking = 790/20
= 39.5 minutes
3A
3B
3. measuring temperature
3. The finger from the hot water felt cold when placed in the room temperature water.
4. The finger from the cold water felt hot when placed in the room temperature water.
5. The results suggest that the fingers detect whether they are gaining or losing heat rather
than the actual temperature of an object.
2. The food colouring quickly spread throughout the glass of hot water, took a few minutes to
colour the warm water and moved very slowly in the cold water. The particles in the hot
water are moving much faster than the warm tap water and even more so than those in the
cold water. Therefore they collide with the colour particles much more quickly and help
spread the food colouring throughout the water.
64 SC1041 © te aho o te kura pounamu
answer guide
3C 1. A. The bucket of tap water has more heat energy because it contains many more particles.
2. ‘Degree of hotness’ is the measure of the temperature of an object. It is measured in degrees
Celsius (centigrade) ºC, or by the Kelvin temperature scale K.
3. True. ‘Degree of hotness’ depends on the average kinetic energy of the particles and kinetic
energy is related to the speed of the particles or how fast they are moving.
4. True. The ‘quantity of hotness’ or heat energy is the total internal energy of the particles
(molecules) that make up the object. It is the combined kinetic and potential energies of the
particles. Both of these energies are dependent on the mass of the particles (molecules) and
kinetic energy is also related to the speed of the particles, so the statement is true.
5. The water particles absorb heat energy from the stove element and start to move faster and
faster. The temperature of the water increases until the water boils.
4A
4B
4. matter
You will have your own words here, but you should have noted that the sugar crystals feel hard and sharp, the water feels soft and ‘squishy’ and the air-filled bag feels very soft and has no particular shape.
You can’t move your hand around very fast! In fact, the faster you thrash around, the more like a solid the gooey stuff becomes. Sometimes it feels like a solid and other times it feels like a liquid.
4C 1.
Metal Drink
2. States of matter
Liquid
Gas
Solid
Gas
What the molecules are doing
Molecules are loosely packed and far enough apart to slide over one another.
Molecules are free from each other and rapidly moving.
Molecules are tightly packed and vibrating about a fixed position.
© te aho o te kura pounamu SC1041 65
answer guide
3. a. Solids have a definite shape because the particles are tightly packed and vibrating about
a fixed position. Liquid particles are, however, loosely packed and free to slide over one
another.
b. Particles in gases are very far apart and move freely, which allows them to fill any
container they are in; whereas solids and liquids are packed more closely together. In
solids the particles vibrate about a fixed position so they have a definite shape. In liquids
the particles are loosely packed and have a definite volume but not shape.
5A
5B
5. conduction
a. The pieces of butter will begin to melt at the candle end first and then towards the
b. Heat travels from the hot end to the cold end.
a. Metal spoon, plastic spoon.
b. Heat travels faster in metals than plastic or wood.
5C Because the metal knife is a good conductor of heat and it conducts heat away from your body.
5D
5E
1. Copper and silver; cork and wood.
2. The metal silver is a very good conductor of heat and the spoon will get hot very quickly.
1. Cooking pots, oven trays, roasting dishes are all made from metal because they conduct heat
well. Plastic spoons or spoons with plastic handles and wooden spoons, ladles and spatula
are good for stirring hot things because they are poor conductors of heat.
2. Conduction is the movement of heat through materials by the collision of particles. At the
place where heat is supplied, the particles start vibrating faster. These particles collide with
the slower vibrating particles of the cooler side and give up some of their energy to them.
The slower particles gain more heat energy and collide with other particles adjacent to them.
3. To conduct, the particles need to stay in a fixed position and vibrate. Particles in the water
are free to move. When heated they move away from the heating source.
4. a. Copper, iron, glass, wood.
b. Cork is a poor conductor of heat; it will protect the table top from heat damage.
66 SC1041 © te aho o te kura pounamu
answer guide
6A
6B
6C
6. insulators
Even though both the wooden chopping board and the metal tray are at the same temperature you will feel that the metal tray is colder than the wooden board. This is because metal conducts heat away from the body faster than the wood, which is a poor conductor.
3. It depends on how thick the materials are. One possible answer is: aluminum foil, paper,
cardboard, tea towel, polypropylene jacket.
1. Particles in gases are very far apart, which prevents collisions between them, and they are
therefore very poor conductors of heat energy.
2. The air particles will no longer be trapped between the woollen fibres and so will not act as
an insulator.
3. Gloves trap air between the hands and the material. This stops body heat escaping into
the air.
4. The newspaper, because it traps air between the food and the paper, which stops the
conduction (and convection) of heat energy away from the food.
7A
7B
7C
7. convection
The un-popped corn kernel sinks to the bottom of the glass while the popped corn floats even though their mass is not changed. When the corn is popped it expands, the particles in the corn are further apart than the corn kernel. The same mass is spread out to a larger space so its volume increases. This makes them less dense.
The ice melts and the coloured water begins to sink.
The cold particles in ice are closer together and they are denser than the particles in the warm water. Denser cold particles therefore sink.
You will see that the coloured water from the bottle begins to rise.
This is because the heat causes the water particles to spread out (expand) and they become less dense than the cooler water at the top. The warmer water, being less dense, rises to the top.
© te aho o te kura pounamu SC1041 67
answer guide
7D 1. a. It is placed at the bottom so that when cold water is heated it will rise and is replaced by
cold water. This will ensure that cold water is continuously heated.
b.
c. The heated water at the bottom will immediately rise to start a convection current. This
speeds up the warming process of the water and setting up a convection current in the jug.
2. a. A convection current is a flow of air or water caused by heating part of the air or water.
Heating part of a body of air or water causes its particles to gain kinetic energy. Then they
move faster and further apart. This means that part of the air or water expands. It be
comes less dense and floats upwards. Denser air or water drops downwards to fill the
space vacated. It in turn is heated. The whole body of air or water starts to circulate in a
b. Convection current requires movement of particles. Particles in liquids and gases are free
to move and particles in solids are locked into place and cannot move.
3. When the heaters are placed at the top, the air at the ceiling will be heated and will stay hot
due to the fact that hot air is less dense. The cold, denser air at the floor level will remain
cold at the bottom.
4. When the flame is turned on the air inside the balloon warms up and rises as it expands and
becomes less dense. As the hot air rises inside the balloon, it pushes the balloon up in the air.
To lower the balloon, the flame is turned off allowing the air to cool. As the air cools, it
becomes denser as the particles are closer together and the balloon sinks back to Earth.
68 SC1041 © te aho o te kura pounamu
answer guide
8A
8B
8C
8. radiation
4. The black can cooled fastest (if they started at the same temperature and the same amount
of water).
5. Black radiates most heat energy. The faster it loses heat, the faster it cools. Heat loss from
convection and conduction should be exactly the same for both cans because they are made
of the same material and surrounded by air, so the difference must be due to radiation.
6. The black had the highest temperature so it absorbed most radiation.
The black can gained most heat energy because it got to a higher temperature.
The test was fair, and the same amount of conduction and convection would occur for both
cans. Radiation is the only way that heat travels from the Sun to the Earth.
1. The thermometer on the left has a higher temperature. This is because its bulb is covered in
black paper. The cooler thermometer is covered in white paper. The black absorbs more heat
radiation from the Sun than white, so it makes the thermometer hotter.
2. Dull black (warmest), dull metal, shiny black, shiny metal (coolest).
3. a. The black absorbs more heat radiation than the white.
b. At night there is no radiation from the Sun, so they both cool down. The black will cool
down more because it is a better emitter of radiation.
4. Firefighters should wear shiny silver so that they don’t absorb much radiation and don’t
get too hot.
5. Take-ways should be in silver bags. Silver will make heat loss by radiation as small as possible.
© te aho o te kura pounamu SC1041 69
acknowledgements
Every effort has been made to acknowledge and contact copyright holders. Te Aho o Te Kura Pounamu apologises for any omissions and welcomes more accurate information.
Photos:
Electric beater, Rubber band on woman's forehead; Boiling water in a pan; Fingers in water temperature; Bags of water and sugar;
Mixing bowl and glass; Making a gooey mixture; Holding a hot drink; Heating a knife; Holding a large cutting knife;
Hands of conducting surfaces; Holding a wooden rolling pin; Jacket lining; Oven glove lining; A measuring cup and saucer;
Two containers; Heat from a toaster; Lady wrapped in silver foil, all by Joseph Fernandez, © 2011, Te Aho o Te Kura Pounamu, NZ.
Different coloured cans; Heated cans; by Kerry Parker, © 2011, Te Aho o Te Kura Pounamu, NZ.
Thermal radiation; Girls around table, Thermometers; Girls on pedestrian crossing, © Kerry Parker, Wellington, NZ.
Use by permission. Earth, Wikimedia Commons.
Public domain:
Cans and Water Cans, © Te Aho o Te Kura Pounamu, NZ.
Tranz:
James Prescott – 0007937
Anders Celsius – 0012382 iStock:
Cover: Boiling water in a pot – 6018713, Women being pulled by dog – 8859560, Flowing water – 14344598
Running boy – 5980080, Black speaker – 4738942, Boy sliding downhill – 9429733, Flashlight – 1353115
Car with lights on – 2313892, Water ripple – 1567876, Paperclip – 5318932, Candle burning – 2174273
Toast – 9935893, Hot water unit – 10483220, Kettle and water boiling – 14026062, Lake – 13965212
Lightbulb – 1754627, Kettle – 9619085, Fireplace – 13831300, Petrol storage – 15369297, Stacked pans – 14205706
Frying pan – 10770174, Polystrene cups – 13281219, Popcorn – 11914896, Popcorn kernals – 396222, Seaview – 15416464,
Radiator – 14715932, Fridge – 12056884, Campfire – 14391764, Sugar – 11003246
Photo Library:
Thermal car – 16231763
SC1041A iStock:
Iron – 278656, Cyclist – 1053660, Lamp – 8490550, Women on phone – 3177547, Boy on swing – 4139256
Birds flying – 1094830, Bird diving – 1094830, Pan on stove – 3942219
70 SC1041 © te aho o te kura pounamu
© te aho o te kura pounamu
self-assessment
sc1041
Fill in the rubric by ticking the boxes you think apply for your work. This is an opportunity for you to reflect on your achievement in this topic and think about what you need to do next. It will also help your teacher.
Write a comment if you want to give your teacher more feedback about your work or to ask any questions.
Fill in your name and ID number.
Not yet attempted
Didn’t understand
Explain the difference between heat and temperature in terms of kinetic energy of particles in a substance.
Explain the arrangement and behaviour of particles in the three different states of matter.
Explain the heat transfer process, conduction, convection and radiation.
Explain how conduction, convection, and radiation occur in nature and how these concepts are used in designing home appliances.
Please place your comments in the relevant boxes below.
Student comment
Explain the difference between heat and temperature in terms of kinetic energy of particles in a substance.
Explain the arrangement and behaviour of particles in the three different states of matter.
Explain the heat transfer process, conduction, convection and radiation.
Explain how conduction, convection, and radiation occur in nature and how these concepts are used in designing home appliances.
Any further student comments.
Understood some
Understood most
Very confident in my understanding
© te aho o te kura pounamu
Phone, fax or email your teacher if you want to talk about any of this work.
Freephone 0800 65 99 88 teacher use only
Please find attached letter
Teacher comment
© te aho o te kura pounamu
cover sheet – sc1041 students – place student address label below or write in your details.
Full Name
ID No.
Address
(If changed) authentication statement
I certify that the assessment work is the original work of the student named above.
Signed
(Student)
Signed
(Supervisor) for school use only assessment www.tekura.school.nz