Document
advertisement

Thermodynamics
Heat Content and
the First
Law Gases)
(Mostly
of Ideal
Dh
Mechanical Energy: Work and Heat
Vacuum Pump
w p Dh
Dh
Ideal Gas: Diluted ensemble of N structure-less,
independent particles with macroscopic, observable
properties described by an Equation of State.
(Sole interactions: Elastic scattering)
When enclosed in a container of volume V, IG exerts a
measurable pressure p on the container walls.
p V N R T
extensive variable
Compression and decompression of a gas volume
(N=const.) change its (kinetic-) energy content U.
U = capacity to perform work w. Charged particles with
structure (atoms,...): additional work types (wel , rxns).
U of gas can be changed by heating or cooling = transfer
of disorganized energy = “heat” q (motion of particles in
container walls).
1. Law of Thermodynamics
(Conservation of Energy in isolated system):
p Force Area ( pext )
incomplete differentials
dU dq dw dq p dV ...
Dw F Dh p DV |
DV 0 : Expansion
DV 0 : Compression
real
gases
Sign Convention: Work and heat are counted positive (w, q > 0), when they increase
the internal energy U of the gas, and negative when done or emitted by the gas.
Random (IG) Velocity/Kinetic-Energy Spectra
dP u
dP(u)/du (a.u.)
4
dP
d
Velocity u (km/s)
dP()/d (a.u.)
u dP u
2
kBT
4
e
3
3 /2
m d u
kBT
Maxwell
Boltzmann
Mean thermal energy
3
kBT
2
Plausibility of EOS (Gas Law) p V N kB T :
N =# of particles colliding with a wall
<u>
momentum transfer to wall per particle
<u>
2
pressure on wall p <u>
T (see EOS)
Kinetic Energy (10-20 J)
Transition
Lattice
Real Gas
Ideal Gas
W. Udo Schröder, 2015
Bound Lattice low T
Unbound Gas, T=300K
LatticeFluid/Real Gas
i (t)/kB (i=1,..,1000)
i (t)/kB
Energy: Science, Technology, Society
32
m
mu2
2
IG particles (mass m) :
4
u
exp
du
2
k
T
B
2kBT
dP u
8kBT
Mean thermal speed u du u
0
du
m
Heat Flow
Heat conduction, flux=current density through area A
jq dQ dt / A
5
T
T
T
Fourier ' s Law : jq T r
i
j
k
x
y
z
Thermal conductivity (W mK )
Energy: Science, Technology, Society
Heat convection: Heat transfer via mass flow
dQ
h A T Tambient
dt
Heat transfer coefficient h (W m2K )
Newton ' s Law of cooling
Heat radiation: Heat transfer via elm. photons
Stefan Boltzmann Law
4
Radiated thermal flux jQ SB T 4 Tambient
Emissivity (often 1)
Stefan Boltzmann constant SB 5.6703 108 W m2K 4
W. Udo Schröder, 2015
The Ideal-Gas Equation of State
p·V = n·R·T;
T U
n=# moles; equivalent: p·V = N·kB·T (N=# particles)
Interacting only via elastic scattering, no bonding Only gas phase!
6
p(V,T)= n R T/V
{p, V, T}
Ideal Gas Constant R
R = 0.0821 liter·atm/mol·K
Energy: Science, Technology, Society
A
R = 8.3145 J/mol·K
R = 8.2057 m3·atm/mol·K
R = 62.3637 L·Torr/mol·K or
L·mmHg/mol·
Boltzmann Constant kB
kB= 1.381·10-23 J/K
State “functions:” {p, V, T} (N=const.). Molar {p, V, T} hyper-plane (monotonic)
contains all possible gas states A. Set of “independent coordinates.” There are no other
states of the gas. Other state functions can be expressed in {p, V, T}.
W. Udo Schröder, 2015
Reversible Processes
Energy: Science, Technology, Society
7
T
Of interest for cyclic
machines.
Slow, equilibrium
processes A B,
subject to boundary
conditions of:
1. Dp = 0
(isobaric)
2. DV = 0
(isochoric)
3. DT = 0 (isothermal)
q=0
4.
q=0
(adiabatic)
follow well-defined,
constrained routes in the
{p, V, T} hyper-plane of
states. Can easily be
inverted reversible
processes.
Reversibility is not guaranteed for all processes involving an ideal gas.
W. Udo Schröder, 2015
Transitions Between States
8
Process 1
A B
along Path 1
Energy: Science, Technology, Society
A
Two different states
B
(A and B)
of the same gas.
Process 2
A B
along Path 2
State functions p, V, T,… describe the system states but not the processes connecting
states. Two states A, B can be reached by different processes representing different
1
2
B and A
B
pathways on the {p,V,T} hyperplane. The two processes A
AB differ by different relative amounts of energy transfer via absorption of heat and work.
W. Udo Schröder, 2015
Reversible Isothermal Expansion/Compression
w = - area under curve p(V)
Total work (1 2):
Use p V R T for expanding 1 mole
2
9
w p (V ) dV R T
Energy: Science, Technology, Society
1
2
1
dV
V
V
R T ln 1 0
V2
w 0 implies system does work
on surroundings
But DU DT 0 q 0 (absorbs heat )
Intersection of {p,V,T} hyperplane with plane T=const.
W. Udo Schröder, 2015
1. Law of Thermodynamics :
V
q DU w w R T ln 1 0
0
V2
Reversible Isobaric Compression
Compress 1 mole at p=const.
Work done on system :
2
2
w p (V )dV p dV 0
1
1
10
p DV R DT > 0 Shaded Area
EOS
Heat transfer
DT 0: system also cools by emitting
5
p DV
5
R
w
2
R
2
Total
heat(transfer
(1 2)
Enthalpy
change
for p const
.) :
Energy: Science, Technology, Society
q C p DT
DH C p DT C p [T2 T1 ] q 0
DV
emitted heat (internal energy )
DU q w (C p R) DT ( DH )
Internal energy change
CV [T2 T1 ] 0
Inverse process: heating at constant p,
e.g., p=patm , leads to
expansion, V2 V1>V2 drives piston out of its cylinder.
W. Udo Schröder, 2015
Reversible Decompression
Isochoric (V = const.) decompression
of 1 mole w =-pDV=0
11
q CV DT 0
Work done on system
w0
Heat transfer
But DU 0, system emits heat
q CV DT CV [T2 T1 ]
Total energy change (1 2)
Energy: Science, Technology, Society
1. Law of Thermodynamics :
DU q w q CV [T2 T1 ] 0
Enthalpy change
DH DU D ( pV ) (CV R) DT
C p [T2 T1 ] (always DH C p DT )
BUT : DH q ( since p const )
Inverse process: heating at constant V, leads to increased temperature
and pressure.
W. Udo Schröder, 2015
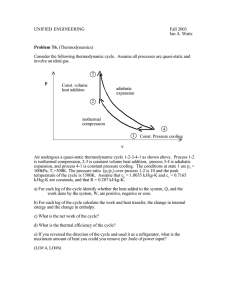
Expansion-Compression Cycles
Observation: IG systems absorbing external (random) heat can produce mechanical work
on surroundings (engine). Continuous operation requires cyclic process (in p-V-T space).
1
work dw=-p·dV
2) Isochoric decompression at V2=const.,
12
Energy: Science, Technology, Society
1) Isothermal expansion at T1=const.
3) Isothermal compression at T2 =const.
4) Isochoric compression V1=const.,
T1
4
2
T2
V2
In one cycle the gas absorbs net heat
energy and does net work,
w = w1 + w2 = -q = CV∙[T2-T1]
Not all absorbed heat is converted,
some has to be dumped as waste heat.
W. Udo Schröder, 2015
1) gas does work
2) gas emits heat
3
V1
Energy balance:
w1 = - q1; DU = 0
q < 0;
DU < 0
3) gas receives work
w 2 = - q2 ;
DU = 0
4) gas absorbs heat
q > 0;
DU > 0
Total energy change:
Total work done:
Total heat absorbed:
DU = 0 (cyclic)
w = w1 + w2 < 0
q = q1+ q2=-w > 0
Thermal Engines: Principle of Operation
p
Net work
done by gas
T1
13
q1
Make an arbitrary cyclic process out of
elementary isothermal and isochoric
processes
Heat energy q1 is absorbed at a high
temperature(s) T1, and partially
Energy: Science, Technology, Society
dumped, |q2| < |q1|, at a lower
q2
T2
V
Horizontal paths traveled in both
directions do not contribute net work
Area within closed p-V paths =
total work done in cyclic process.
temperature(s) T2.
The difference (q1 + q2)= q1-|q2| is
converted into useful work w < 0 done
on surroundings by the gas.
Random heat energy is converted into orderly collective energy
(work, pushing a piston, turning a wheel) !!!!!!! Practical use
W. Udo Schröder, 2015
Work/Heat in Reversible vs. Irreversible Processes
A
rev
14
B
irrev
pext
System interacts with environment, is not isolated
(DT=0).
pext
In process A B, carried out so that system is
always at equilibrium (e.g, pext dV= pgasdV+q),
system produces maximum work.
(balance by including the –sign, sign convention!):
pext
A’
X
wrev < wirrev |wrev |> |wirrev|
pgas
A
A’
irrev
decompresion
Energy: Science, Technology, Society
Opening valve t0 Expansion
Where did the difference Dw go ? Nowhere!
Also less/no heat absorbed on irreversible path.
1. Law TD, and since U is a state function,
If DUAB = DUA’B (q+w)rev = (q+w)irrev
B’
pext
B
V
wrev < wirrev
Wrev is largest amount deliverable (negative) reversibly
qrev is largest amount of heat system can absorb reversibly
and convert into work.
Irreversible, spontaneous processes:
Less efficient conversion of absorbed heat into useful work.
W. Udo Schröder, 2015
qrev > qirrev
Entropy: The Arrow of Time
A small volume Vin containing randomly moving (thermal) particles “expands,” or a cluster
of many particles disintegrates, particles diffuse into and occupy all available states W.
Vin
T
j, i
time
S ≈ Smax
Spontaneously from simple
to ever more complex.
Essentially irreversible.
Vfin
T
S ≈ Smax
2. Law of TD : # of occupied states (W) never decreases
S : k B n W k B pij n pij increases (" Entropy ")
i, j
W
In principle, all transitions are individually reversible.
But: In the aggregate, it is unlikely that all particles move back to
exactly the same positions (states) from which they came. Probability
for complete reversal is non-zero but very small.
Poincaré Recurrence Time: trec ~ W
Number (W ) of occupied
Only equilibrium (random) states are equivalent,
states increases in time.
connected by reversible processes.
Carnot Cycles
p
Adiabatic (q 0) EoS
(p1, V1)
T V
16
q=0
1
Energy: Science, Technology, Society
(p2, V2)
work
q=0
T2
DS1
(p3, V3)
q1
q
2 DS2
T1
T2
Entropy is conserved in reversible
cyclic processes : DS1 DS2 0.
S state function (descriptor )
q1 T1 DS1
q2 T2 DS2
q AB
T
sign for reversible A B only.
For any process : DS AB
W. Udo Schröder, 2015
c p cV
T1
(p4, V4)
" Entropy "
const;
V
Similar to previous examples:
Isothermal expansion at Th=T1
Adiabatic expansion Th Tc=T2
Isothermal compression at Tc <Th
Adiabatic compression Tc Th,
Adiabatic works cancel.
Adiab. expansion/compr.
V4/V1= V3/V2 V4/V3= V1/V2
Energy balance: w = q1 + q2 > 0
on isothermal portions:
q1 w1
V2
V1
V
p dV R T1 ln 2 0
V1
1 V
V4
V
q2 w2 pdV R T2 ln 4 0
V3
V3
w0
Reversible adiabatic exp./compr.: DS = q/T= 0
since q= 0.
Irreversible adiabatic exp./compr.: DS > 0.
Efficiency of Carnot Engines
Theoretical Carnot efficiency
Th
C
Energy: Science, Technology, Society
17
qh= DS·Th
DS = const
C 1
-w = qh+qc=DS·(Th- Tc)
qc= -DS·Tc
Tc
w qh qc
qh
qh
qc
T
1 c
1
Th
qh
Th
U const
Process at p, T const.
q DH
T T
w Th Tc DS h c Th DS
Th
T T
h c DH Th D H T S
Th
DG
In practice, Th depends on fuel heating value (max temperature Tad).
Transfer from fuel to hot reservoir: F Tad Th Tad Tc
Effective Carnot efficiency:
W. Udo Schröder, 2015
C F 1
Tc
Th
Tad Th
T
T
ad c
Entropy Flow in Carnot Engines
p
(p1, V1)
Th
18
q=0
qh= DS·Th
Th
(p2, V2)
work
(p4, V4)
q=0
Energy: Science, Technology, Society
Tc
(p3, V3)
Hydrodynamic Power Plant
Inlet
Reservoir
Turbine
-w = qh+qc=
= DS·(Th -Tc)
V
DM∙g∙h1
DM∙g∙h2
qc= -DS·Tc
Tc
Entropy DS from the hot reservoir
enters the engine with a heat energy
of DS·Th,
produces work and leaves it again
with a heat energy of DS·Tc,
which is dumped into the cold sink.
Outlet
Analog: Stream of water DM from a reservoir carries energy DM∙g∙h1 , enters a hydroturbine, produces work, and leaves with an energy DM∙g∙h2 , which is dumped into the
river.
Mass flow j dM dt . Entropy flow j dS dt
M
W. Udo Schröder, 2015
S
Steady-Flow Processes
1. Law of Thermodynamics
E U
19
(Conservation of total energy in isolated system):
M= mass, = velocity, Vpot =potential energy (often ≈0)
Mass density r = rm (kg/m3), homogeneous
Internal energy density u (J/m3)
Enthalpy density h = u + p
Internal energy
Kinetic energy
dUi ui (A i dx i )
dK i 1 2 ri i2 (A i dx i )
1
M 2 V pot
2
uo , ro , o
dVo
dVi A i dx i
Energy: Science, Technology, Society
Mechanical work dWi pi (A i dx i )
Carried by mass flow through system
incoming
outgoing
1
rii2 (A i dxi )
2
1
dUo uo (A o dxo ) po (A o dxo ) r0o2 (A o dxo )
2
dVi
ui , ri , i
dUi ui (A i dx i ) pi (A i dx i )
Mass conservation :
dMi r A i r A o dMo
Additional mechanical work output dW , heat output dQ
dE d Uin Uout dW dQ
dE
Steady state :
0
dt
dt
dt
dt
dt
dW dQ dW
1
1
hi rii2 A i i ho roo2 A o o
dt
dt
dt
2
2
well insulated
W. Udo Schröder, 2015
Power
Fuel Flow
2
dW hi i2 ho o dM
dt
2 ro
2 dt
ri
Energy: Science, Technology, Society
20
Spontaneous Reactions Require Free Energy
G
W. Udo Schröder, 2015
W. Udo Schröder, 2015
Energy: Science, Technology, Society
21