τ τ τ τ τ τ
advertisement
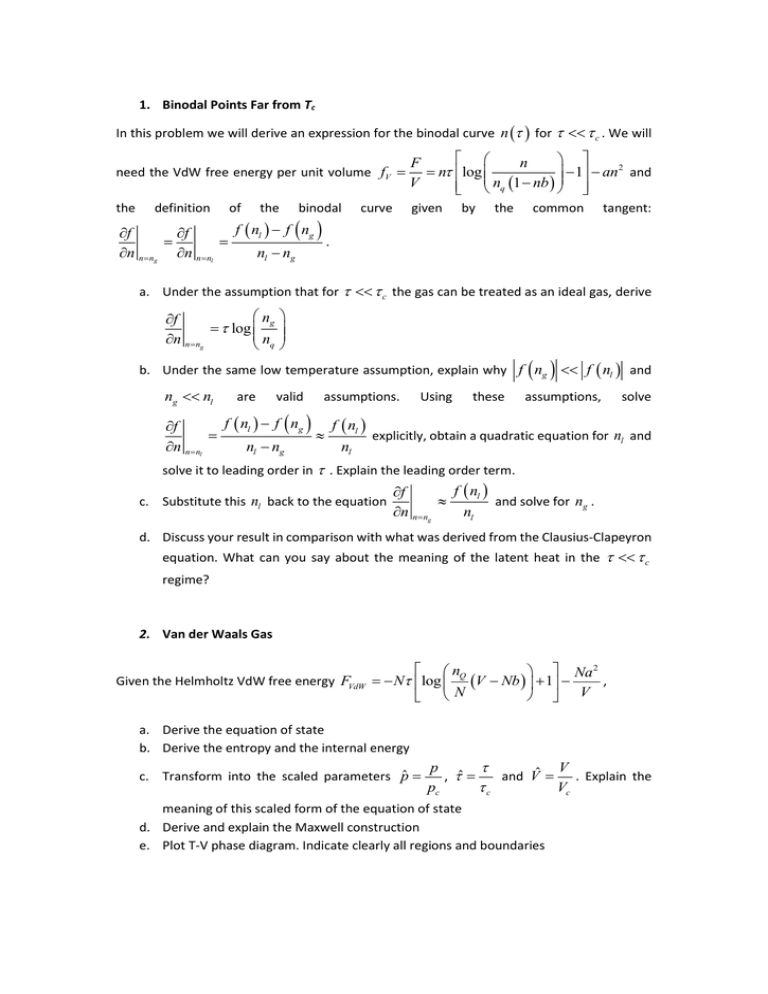
1. Binodal Points Far from Tc In this problem we will derive an expression for the binodal curve n for c . We will need the VdW free energy per unit volume fV F n 2 n log 1 an and V n 1 nb q the given definition of the binodal f nl f ng f f . n n ng n n nl nl ng curve by the common tangent: a. Under the assumption that for c the gas can be treated as an ideal gas, derive ng f log n n n ng q b. Under the same low temperature assumption, explain why f ng f nl and ng nl are valid assumptions. Using these assumptions, solve f nl f ng f nl f explicitly, obtain a quadratic equation for nl and n n nl nl ng nl solve it to leading order in . Explain the leading order term. c. Substitute this nl back to the equation f nl f and solve for ng . n n ng nl d. Discuss your result in comparison with what was derived from the Clausius-Clapeyron equation. What can you say about the meaning of the latent heat in the c regime? 2. Van der Waals Gas Given the Helmholtz VdW free energy FVdW nQ Na 2 , N log V Nb 1 V N a. Derive the equation of state b. Derive the entropy and the internal energy c. Transform into the scaled parameters pˆ V p , ˆ and Vˆ . Explain the Vc c pc meaning of this scaled form of the equation of state d. Derive and explain the Maxwell construction e. Plot T-V phase diagram. Indicate clearly all regions and boundaries