Physics 142: Lecture 8 Today`s Agenda REVIEW: The Ideal Gas
advertisement

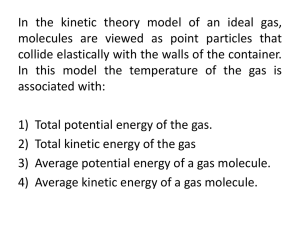
Physics 142: Lecture 8 Today’s Agenda z REVIEW: The Ideal Gas Law z Temperature and Kinetic Energy Î Review: Ideal Gas Law (10.3) Î Review: Thermal Expansion (10.4) Î Kinetic Theory of Gases (10.5) ÎN = number of molecules ÎT = absolute temperature (K) Suggested problems for Ch-10: 35, 52, 56,59,79, 80 Midterm: Friday, Feb 16, 2007 12:25 – 13:25 PV = NkBT z Alternate way to write this z PV = nRT În = number moles THEATER AUDITORIUM Assignment 1 is due this Wednesday, Jan 24 ÎR = ideal gas constant = 8.31 J/(mol-K) P1 V1 P2 V2 = T1 T2 if n or N remains the same Lecture 8, Pg 1 Lecture 8, Pg 2 Expanding Rods Thermal Expansion z z When temperature rises Îthings tend to expand amount of expansion depends on… Îchange in temperature Îoriginal length Îcoefficient of thermal expansion » L0 + ∆L = L0 + α L0 ∆T » ∆L = α L0 ∆T (linear expansion) » ∆V = β V0 ∆T (volume expansion) Temp: T L0 Temp: T+∆T ∆L Lecture 8, Pg 3 Lecture 8, Pg 4 Example Amazing Water As you heat a block of aluminum from 0 C to 100 C its density 1. Increases 2. Decreases 3. Stays the same z T = 100 C T=0C Water is very unusual in that it has a maximum density at 4 degrees C. That is why ice floats, and we exist! 1000.00 999.95 999.90 999.85 999.80 999.75 999.70 Density 999.65 999.60 999.55 0 2 4 6 8 10 Lecture 8, Pg 5 Lecture 8, Pg 6 Example Example Not being a great athlete, and having lots of money to spend, Gill Bates decides to keep the lake in his back yard at the exact temperature which will maximize the buoyant force on him when he swims. Which of the following would be the best choice? 1000.00 (1) 0 C 999.95 (2) 4 C 999.90 999.85 (3) 32 C 999.80 Density (4) 100 C 999.75 999.70 (5) 212 C An aluminum plate has a circular hole cut in it. An aluminum ball (solid sphere) has exactly the same diameter as the hole when both are at room temperature, and hence can just barely be pushed through it. If both the plate and the ball are now heated up to a few hundred degrees Celsius, how will the ball and the hole fit ? 1. The ball wont fit through the hole any more 2. The ball will fit more easily through the hole 3. Same as at room temperature 999.65 999.60 999.55 0 2 4 6 8 Lecture 8, Pg 7 10 Lecture 8, Pg 8 Why does the hole get bigger when the plate expands ??? Imagine a plate made from 9 smaller pieces. Each piece expands. If you remove one piece, it will leave a “square” hole Expansion Act An aluminum plate has a circular hole cut in it. An iron ball (solid sphere) has exactly the same diameter as the hole when both are at room temperature, and hence can just barely be pushed through it. If both the plate is cooled in liquid nitrogen, 1. The ball wont fit the hole any longer 2. The ball will fit more easily 3. Same as at room temperature Object at temp T Lecture 8, Pg 9 Kinetic Theory of Gases: The relationship between kinetic energy and temperature for monatomic ideal gas 2 vrms 1 N = ∑ vi2 n i =1 1 2 pV = Nmvrms 3 pV = NkbT “rms” stands for “root-mean-square” 1 2 Nmvrms = NkbT 3 Lecture 8, Pg 10 Example Suppose you want the rms (root-mean-square) speed of molecules in a sample of gas to double. By what factor should you increase the temperature of the gas? 1. 2 2. 2 3. 4 1 2 3 mvrms = kbT = K , where K is average kinetic energy per molecule 2 2 m is the mass of one molecule Lecture 8, Pg 11 Lecture 8, Pg 12 Problem Internal Energy of Ideal Monatomic Gas (a) What is the average kinetic energy per molecule of an ideal gas at a temperature of 27°C ? (b) What is the average (rms) speed of the molecules if the gas is helium? (A helium molecule consists of a single atom of mass 6.65x10-27kg) 1 3 2 2 mvrms = 2 kbT = K The total internal energy of an ideal gas is equal to the total kinetic energy of all the molecules 3 3 3 ⎛1 2 ⎞ U = N ⎜ mvrms ⎟ = N kbT = ( nN A ) kbT = n ( N A kb ) T 2 2 2 ⎝2 ⎠ 3 = nRT 2 U= 3 nRT 2 total internal energy Lecture 8, Pg 13 Lecture 8, Pg 14 Summary Kinetic Theory Ideal Diatomic Gas z Average Kinetic Energy (per molecule) of any Ideal Gas Î z 2 K = 1 2 mvrms = 3 2 kbT Total Internal Energy of an Ideal Diatomic Gas Î z U= 5 nRT 2 z Temperature is a measure of the average Kinetic Energy of molecules Average Kinetic Energy (per molecule) of any Ideal Gas Î z Total Internal Energy of an Ideal Monatomic Gas Î z U= 3 nRT 2 Total Internal Energy of an Ideal Diatomic Gas Î Lecture 8, Pg 15 2 K = 1 2 mvrms = 3 2 kbT U= 5 nRT 2 Lecture 8, Pg 16