( ) ln - Department of Chemistry
advertisement
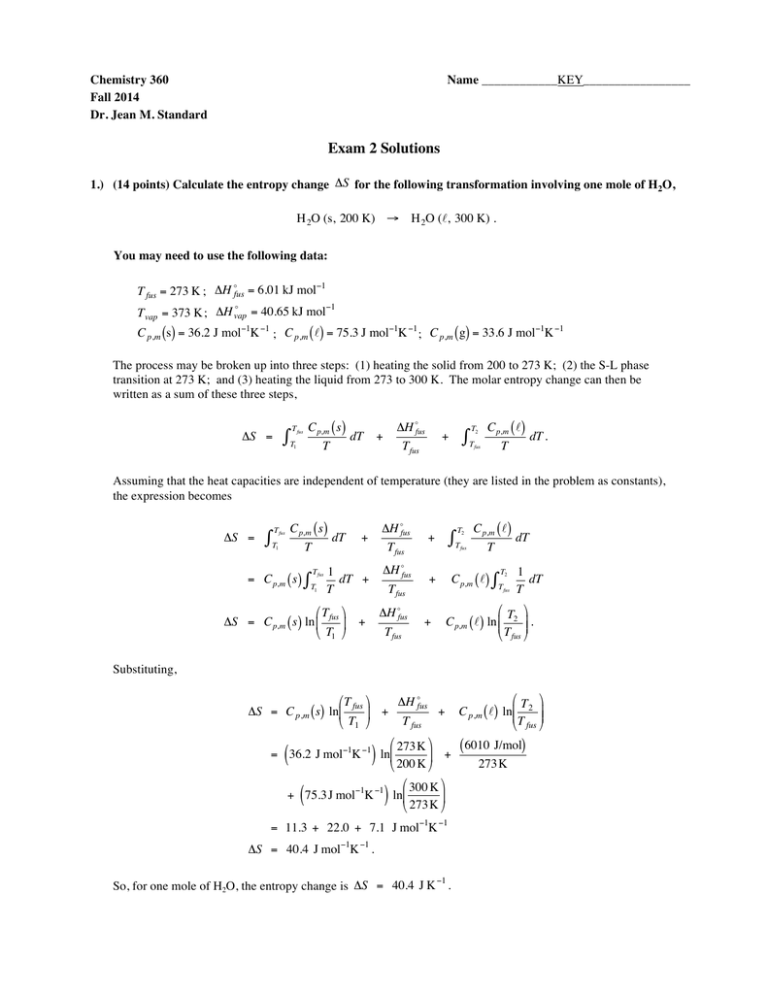
Chemistry 360 Fall 2014 Dr. Jean M. Standard Name ____________KEY_________________ Exam 2 Solutions 1.) (14 points) Calculate the entropy change ΔS for the following transformation involving one mole of H2O, H 2O (s, 200 K) → H 2O (ℓ, 300 K) . € You may need to use the following data: € ! −1 T fus = 273 K ; ΔH fus = 6.01 kJ mol ! −1 T vap = 373 K ; ΔH vap = 40.65 kJ mol € € € C p,m (s) = 36.2 J mol−1K −1 ; C p,m ( ℓ) = 75.3 J mol−1K −1 ; C p,m ( g) = 33.6 J mol−1K −1 € The process € may be broken up into three steps: (1) heating the solid from 200 to 273 K; (2) the S-L phase transition at 273 K; and (3) heating the liquid from 273 to 300 K. The molar entropy change can then be € written as a sum of these€three steps, ΔS = T fus ∫T C p,m ( s) T 1 dT + ΔH !fus T fus + ∫ T2 C p,m ( ℓ) T fus T dT . Assuming that the heat capacities are independent of temperature (they are listed in the problem as constants), the expression becomes ΔS = T fus ∫T C p,m ( s) T 1 = C p,m ( s) ∫ T fus T1 dT + ΔH !fus T fus + T2 ∫T fus C p,m ( ℓ) T dT ΔH !fus T2 1 1 dT + + C p,m ( ℓ) ∫ dT T fus T T T fus #T & #T & ΔH !fus ΔS = C p,m ( s) ln % fus ( + + C p,m ( ℓ) ln %% 2 (( . T fus $ T1 ' $ T fus ' Substituting, # T fus & ΔH !fus ΔS = C p,m ( s) ln% + ( + T fus $ T1 ' # 273 K & = 36.2 J mol−1K −1 ln% ( + $ 200 K ' # 300 K & + 75.3 J mol−1K −1 ln% ( $ 273 K ' ( ) ( ) = 11.3 + 22.0 + 7.1 J mol−1K −1 ΔS = 40.4 J mol−1K −1 . −1 So, for one mole of H2O, the entropy change is ΔS = 40.4 J K . € € #T & C p,m ( ℓ) ln%% 2 (( $ T fus ' ( 6010 J/mol) 273 K 2 2.) (14 points) The standard molar enthalpy of formation of Fe2O3 (s) is ΔH !f standard molar enthalpy of formation of SO2 (g) is ΔH !f = −824.2 kJ/mol , and the = −296.8 kJ/mol (both at 298 K). Use this information, along with the standard molar enthalpy change of the following reaction at 298 K, € ΔH R! = −1655 kJ/mol, 2 FeS2 (s) + 112 O 2 (g) → Fe € 2O3 (s) + 4 SO 2 (g) to determine the standard molar enthalpy change of the reaction shown below at 298 K: Fe (s) + 2 S (s) → FeS 2 (s) . [Note: The standard state of iron is Fe(s) and the standard state of sulfur is S(s) at 298 K.] € The formation reaction for Fe2O3 is 2 Fe (s) + 3O 2 2 (g ) → Fe 2O3 (s) . The formation reaction for SO2 is S (s) + O 2 (g) → SO 2 (g) . The desired reaction can be constructed from the reactions given in the problem in the following way: 2 1 2 1 2 [ [ S (s) [2 Fe (s) + O 2 ( g) → SO 2 ( g) + 3 O 2 2 → Fe 2O 3 (s)] ( g) Fe 2O 3 (s) + 4 SO 2 (g) Fe (s) + 2 S (s) ] → 2 FeS 2 (s) + 11 2 O 2 (g) ] FeS 2 (s) → From the combination of reactions above, the enthalpy of reaction can be calculated from Hess' Law, € ΔH !f ( FeS 2 ) = 2ΔH !f (SO 2 ) + 12 ΔH !f ( Fe 2O 3 ) − 12 ΔH r! , ! where ΔH r corresponds to the standard molar enthalpy of the reaction between FeS2 and O2 given in the € problem. Substituting, ΔH !f ( FeS 2 ) = 2ΔH !f (SO 2 ) + € 1 2 ΔH !f ( Fe 2O 3 ) − = 2(−296.8 kJ/mol) + ΔH !f ( FeS 2 ) = − 178.2 kJ/mol. € 1 2 1 2 ΔH r! , (−824.2 kJ/mol) − 1 2 (−1655 kJ/mol) 3 3.) (14 points) The statistical definition of the entropy is S = kB ℓnW , where kB is the Boltzmann constant and W is the probability. Discuss how this definition along with the Second Law of Thermodynamics lead to the conclusion that spontaneous processes are those that yield states of higher probability. Qualitatively, what is the expected change in probability during the course of a spontaneous process? For a general process occurring between an initial state (1) and a final state (2), the entropy change ΔS determined using the equation above would be #W & ΔS = S2 − S1 = k ℓn % 2 ( . $ W1 ' € If the ratio W2 / W1 is greater than 1, this implies that state 2 has higher probability than state 1. This also means that the logarithm of W2 / W1 is positive and therefore ΔS is positive, ΔS > 0. € This result also implies, of course, that state 2 has higher entropy than state 1. Therefore, there is a correlation between higher probability and higher entropy. € From the Second Law we know that for an isolated system, the entropy increases for a spontaneous process ( ΔS ≥ 0 ); this corresponds to the system moving from a state of lower probability (or lower entropy) to a state of higher probability (or higher entropy). Thus, the probability increases during the course of a spontaneous process. 4 4.) (15 points) True/false, short answer, multiple choice. a.) True or False: For the isothermal reversible compression of an ideal gas, the quantities ΔU and ΔS are predicted to be ΔU = 0 and ΔS < 0 . € € b.) True or False : The Debye equation gives the form of the constant pressure molar heat capacity of a solid at low temperature and has the form C !p,m (T ) = aT 2 . c.) Short answer ____Bomb (or adiabatic)____ calorimetry is carried out at constant volume, whereas ____solution____ calorimetry is carried out at constant pressure. d.) Short answer For two chemical reactions that combine to give a third reaction (the overall reaction), the enthalpy change of the overall reaction (3) is given as ΔH 3 = ΔH1 + ΔH 2 , which is an example of the application of __________Hess' Law_________ . € e.) Multiple Choice: Which of the following corresponds to the correct relationship? !∂ G $ 1) T = # & . " ∂ P %S "∂ U % 2) T = − $ ' . # ∂ V &S "∂ A % 3) T = − $ ' . # ∂ V &S !∂ H $ 4) T = # & . " ∂ S %P 5 #∂ H & ( for a gas obeying the equation of state 5.) (14 points) Determine % $ ∂ P 'T Z = € PVm = 1 + BP + CP 2 , RT where B and C are constants. Since a partial derivative of H is requested, it is helpful to start with the fundamental equation for H, dH = T dS + V dP. Take the partial derivative with respect to V (at constant T) of both sides of the equation, !∂ H $ !∂ S $ # & = T # & + V . " ∂ P %T " ∂ P %T The partial derivative on the right side of the equation cannot be determined from the equation of state. However, one of the Maxwell relations can be used, !∂ S $ !∂ V $ # & = − # & . " ∂ P %T " ∂ T %P Substituting yields !∂ H $ !∂ V $ # & = −T # & + V . " ∂ P %T " ∂ T %P Now, the partial derivative on the right side can be calculated from the equation of state. Since the equation of PV state is Z = m = 1 + BP + CP 2 , solving for Vm gives RT PVm = 1 + BP + CP 2 RT RT Vm = 1 + BP + CP 2 P RT or Vm = + BRT + CRTP . P ( ) Now, converting to V instead of Vm leads to the expression V = € nRT + nBRT + nCRTP . P Taking the partial derivative, !∂ V $ nR # & = + nBR + nCRP . ∂ T P " %P 6 5. Continued Substituting into the expression for the partial derivative of H gives the result, !∂ H $ !∂ V $ # & = − T # & + V " ∂ P %T " ∂ T %p ! nR $ = −T # + nBR + nCRP & + V "P % ! nRT $ = − # + nBRT + nCRTP & + V " P % = −V + V !∂ H $ # & = 0. " ∂ P %T 7 6.) (14 points) Using the values in the table below reported at 25°C, determine hydrogenation of acetylene to ethane, ΔH R! at 400ºC for the C2 H 2 ( g) + 2 H 2 (g) → C2 H 6 (g) . Assume that C p,m is independent of temperature for all species in the reaction. € C2H2 (g) ΔH !f C p,m (kJ/mol) (Jmol–1K–1) 226.73 € C2H6 (g) –84.68 H2 (g) 43.93 € 52.63 0 28.82 The first step is to determine the enthalpy of reaction at 298 K: ΔH R! = ΔH !f (C2 H 6 ) − ΔH !f (C2 H 2 ) − 2ΔH !f ( H 2 ) = −84.68 kJ/mol − ( 226.73 kJ/mol) − 2 ( 0 ) ΔH R! = −311.41 kJ/mol. The temperature dependence of the molar enthalpy of reaction is given by ΔH R! (T2 ) = ΔH R! (T1 ) + ΔC p,m ⋅ (T2 − T1 ). This equation assumes that the molar heat capacities are independent of temperature. In the equation, ΔC p,m is the difference in molar heat capacities between products and reactants with the appropriate signed stoichiometric coefficients included, € ∑ν C ΔC p,m = i p,m (i ) . i For this reaction, the heat capacity difference is € ΔC p,m = ∑ν iC p,m (i ) i = C p,m (C2 H 6 ) − C p,m (C2 H 2 ) − 2C p,m (O 2 ) = 52.63 − 43.93 − 2 ( 28.82 ) Jmol−1K −1 ΔC p,m = −48.94 Jmol−1K −1. Substituting, the molar enthalpy of reaction at 400°C (673 K) is ΔH R! ( 673 K ) = ΔH R! ( 298 K ) + ΔC p,m ⋅ ( 673 − 298 K ) $ 1 kJ ' = −311.41 kJ/mol + −48.94 Jmol−1K −1 ( 673 − 298 K ) & ) % 1000 J ( = −311.41 −18.35 kJ/mol ( ΔH R! ( 673 K ) = −329.76 kJ/mol . ) 8 7.) (15 points) True/false, short answer, multiple choice. a.) True or False : A system consisting of a container filled with 1 mole of C4H10 (g) has lower entropy than a system consisting of the same container filled with 1 mole of CH4 (g), both at 25°C and 1 atm. b.) True or False : The standard state is defined to be the pure form of an element at 1 bar and 25°C. c.) Short answer The ____Second Law of Thermodynamics_____ states that entropy increases for a spontaneous process in an isolated system. d.) Short answer The ______Helmholtz Energy______ is defined as U − TS . e.) Multiple Choice: At 298 K, ΔGR! = –158 kJ/mol for a certain chemical reaction. In addition, this reaction has ΔH R! > 0 . Which of the following is a reasonable expectation for ΔGR! at 600 K? 1) ΔGR! = –195 kJ/mol . 2) ΔGR! = –158 kJ/mol . 3) ΔGR! = –121 kJ/mol . 4) ΔGR! = +35 kJ/mol .