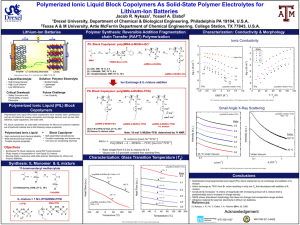
0002-7863 183 I t,s0-s-01 l 5$01.-s010 el
advertisement

('oprright
I t e l r r i n t e t l l r o r n t h e . l , ' r r r n r r lo t t h e . \ t n e r i t a r t ( ' h e n r i t ' i t lS o t ' i t ' t r ' .l 9 l t 3 , / / i ' ) . I l ; .
c l f i s : i i r r t h t , . . \ n r t , r i t i r n ( h t . r r i i t ' a lS o t ' i e t v i l n ( l r e l ) r i n t e c l l x ' p e r m i s s i o t t o f t h e t ' o l r v r i g h t o w n e r .
Preparation of a Mixture of Nucleoside Triphosphates
from Yeast RNA: Use in Enzymatic Synthesis
Requiring Nucleoside Triphosphate Regeneration and
Conversion to Nucleoside Diphosphate Sugarsr
Chi-Huey Wong, Sharon L. Haynie,2and
George M. Whitesides*
Department of Chemistry
MassachusettsInstitute of Technology
Cambridge, Massachusetts 02139
ReceiuedJune 14, 1982
This paperdescribesa practicalprocedurefor convertingyeast
R N A i n t o a m i x t u r eo i A T P , U T P , G T P , a n d C T P ( S c h e m eI ) .
0l
0 0 0 2 - 7 8 6138 3
I t , s 0 - s -l051$ 0 1 . - s 0 1 e
This mixture can be usedas a sourceof nucleosidetriphosphates
for the synthesisof nucleosidediphosphatesugars. Theselatter
synthesesof
are requiredin most enzyme-catalyzed
substances
oligo and polysaccharides.lIn addition,sincemany ATP-utilizing
will also acceptGTP,
enzymes(especiallyphosphotransferases)
UTP, and CTP,a the mixture of nucleosidetriphosphatesserves
* I n q u i r i e ss h o u l db e a d d r e s s etdo G . M . W . a t t h e D e p a r t m e not f C h e m , A 02138.
i s t r y ,H a r v a r dU n i v e r s i t y l,2 O x f o r d S t r e e t ,C a m b r i d g eM
s f H e a l t h ,G M - 2 6 5 4 3a n d G M ( I ) Supported
b y t h e N a t i o n a lI n s t i t u t e o
30367.
(2) National ScienceFoundationPredoctoralFellow.
( 3 ) N i k a i d o ,H . ; H a s s i d W
. h e m .B i o c h e m . 1 9 7 1 ,
, . Z . A d u . C a r b o h y d rC
P. R.;Numez, H. A.; Barker,R. Bia:hemistry1982,
26. 351-483. Rosevear,
t1. l42l-3r
1983 American Chemical Society
1 1 6 J . A m . C h e m .S o c . , V o l . 1 0 5 , N o . l , 1 9 8 3
Communications to the Editor
Scheme Io
RNA
Nuc/eose P1
Y
Oligonucleolrdes
pppose.
ll
Pi
OH
QP0lH
)'"^-
,, [aoe
""2
no^$-_g...
__, irn r___;)_or
+ c D P + cDp + uDpl
\px
HK, acK. Acp
_
larP
,Lcoi
+ c T P + crP + Jr"l
r
/-.-n
'
PPOgE
)
l DP.;D
ppoaH
-" \i_;
-!=-\^
f
'
OH
1l'--\r,O _
u.
=\
tn'
0 P 0 af r
,/7
/, ,v
\
bD- 0H
2?+
[arc
* cre
o PNPasc,
polynucleotide
phosphorylase,
PK, pyruvatekinase;
HK, hexokinase;
AcK, acetatekinase;PGM,phosphoglucomutase;
UDPGP,U DP-glucosep yrophosphorylasc
inorganicpyro; PPase,
phosphatase
; AcP,acetylphosphate.
as a convenientsourceof phosphateequivalentsin other enz y m e - c a t a l y z eodr g a n i cs y n t h e s e s . s
Conversionof RNA to the mixture of nucleosidetriphosphates
i n v o l v e dt w o s t e p s . I n t h e f i r s t , n u c l e a s eP r ( 8 . C . 3 . 1 . 4 . - ,i n
solution)was usedto hydrolyzehigh molecularweight veastRNA
to a mixture of lower molecularweightoligonucleotides.6
In the
(PNPase.E.C. 1.7.7.8.imsecond,polynucleotide
phosphorvlase
m o b i l i z e di n P A N g e l T )c o n v e r t e dt h e s eo l i g o n u c l e o t i d ei sn t o
n u c l e o s i d ed i p h o s p h a t e sb y r e a c t i o n w i t h p h o s p h a t e . T h i s
equilibrium conversionstrongly favorsoligonucleotides.EThc
nucleosidediphosphateswere convertedto nucleosidetriphosphates
by phosphorylationin situ by using phosphoenolpyruvate(PEP)e
and pyruvatekinase(PK, E.C. 2.7.1.40)to drive the reaction.r0
The most expensive enzyme in this synthesis is PNPase
(54.00/unit,Sigma). The initial treatmentby nucleaseP1permits
useof PNPaseimmobilized in PAN gel, in which form the enzyme
is both relativelystable and very easily recoveredfor reuse.
In a representativeprocedure,yeastRNA (15 g, 95% pure, from
BoehringerMannheim) and 50 units of nucleaseP, in 20 mL of
w a t e r ( 0 . 1 m M i n M n C l 2 ,p H 6 . 0 ) w a s s t i r r e da t 5 0 o C f o r I h .
The mixture was adjustedimmediatelyto pH 8.2 by addingcold
( 4 ) B e r g m e y e rH
, . U . I n ' M e t h o d s o f E n z y m a t i cA n a l y s i s " A
; cademic
P r e s s :N e w Y o r k , 1 9 7 4 ;p 2 0 8 1 . C h u n g ,A . E . J . B i o l . C h e m .1 9 6 7 , 2 4 2 ,
1 8 2 - 6 . P l o w m a nK, . M . ; K r a l l , A . R . B i o c h e m i s t r1y 9 6 5 , 4 , 2 8 0 9 - 2 5T. h e s e
phosphoglyccrate
enzymesincludehexokinase,
kinase,ac€tatekinase,pyruvate
kinase,phosphofructokinase,
NAD kinase,glycerokinase,
and glucose lphosphatekinase.
(5) Pollak,A.; Baughn,R. L.; Whitesides,
G. M. J. Am. Chem.Soc.1917,
9 9 , 2 3 6 6 - 7 . R i o s - M e r c a d i l l oV,. M . ; W h i t e s i d e sG, . M . J . A m . C h e m .S o c .
1 9 7 9 ,t 0 r , 5 8 2 8 - 9 .
(6) CommercialyeastRNA containsa high proportionof polynucleotides
with M" 105-106.This materialwas not a substratefor PAN-immobilized
PNPaseand was a poor substratefor this enzymein solubleform. After
digestionwith nucleaseP,, it becamea good substratefor immobilized
PNPase.
( 7 ) P o l l a k ,A . ; B l u m e n f e l dH, . ; W a x , M . ; B a u g h n R
, . L . ; W h i t e s i d e sG
,.
M, J. Am. Chem.Soc. 1980. 102. 6324-36.
(8) Godefroy-Colburn,T.; Grumberg-Manago,M. In "The Enzymes";
B o y e r ,P . D . , E d . ; A c a d e m i cP r e s s :N e w Y o r k , 1 9 7 2 ;V o l . 7 , p p 5 3 3 - 7 4 . A t
20 oC, 80%,of ADP presentinitially is polymerized,correspondingto an
equilibriumconstantK = 16 for the reactionADP + ApA : ApApA + Pi
(AG"' - -RI ln K e -1.7 kcal/mol).
(9) Hirschbein,B. L.: Mazenod,F. P.; Whitesides,G. M. J. Org. Chem.
1982.47. 3't6s-6.
( l0) If the reactionApApA + Pi : ApA + ADP (AG'' = *l.7 kcal/mol)
is coupledwith the reactionPEP + ADP + ATP + pyruvate(.\Go' = -7.5
kcal/mol), the free energy(AGo') for the overallreactionApApA + Pi + PEP
: ApA + ATP is -5.8 kcal/mol. Saber,H. A. "Handbookof Biochemistry"l
1 9 7 0 ,T h e C h e m i c a lR u b b e rC o . : C l e v e l a n dO
, H , 1 9 7 0 ;p p J l 8 0 - 5
5 N NaOH solutionand diluted to a total volume of I L in a
solution that containedpotassiumphosphate(0.2 M), MgCl2
(2mM), PEP (monopotassium
s a l t ,8 0 m M ) , a n d P A N - i m m o b i l i z e dP N P a s e( 8 u n i t si n 2 0 m L o i g e l ) r r a n d P K ( 5 0 u n i t si n
I mL of gel). The reactionmixture was stirred at room temperature under argon for 4 days, and the pH was controlled
of the reactionmixture
between8.2 and 8.5. Enzymaticanalysisr2
indicatedthat it contained30 mmol of nucleosidetriphosphates
(68Voyield basedon RNA); no further increasein the yield of
thesesubstanceswas observedl3with longer reactiontimes. The
gel particleswere removed,the solutionwas adjustedto pH 3.0
at 4 oC by adding cold concentratedHCI with stirring, and a
precipitatewas removedby filtration. The filtrate was adjusted
to pH 8.0 (5 N NaOH) and concentratedunder reducedpressure
at 35 oC to a volumeof 50 mL. This solutioncontaineda mixture
(28 mmol. 62% yieldbasedon RNA)
of nucleoside
triphosphates
in theserelativequantities:roATP, 24%, UTP , 28%: GTP, 30Vo:
CTP, 18%. The enzymatic activities recoveredafter these
transformationswere PN Pase,7 5Vo,and PK, 92%.
obtainedin this proThe mixture of nucleosidetriphosphates
cedureis contaminatedwith a numhr of other components.These
other materialsdo not. however.seemto interferewith the use
of the nucleosidetriphosphatesin further enzymaticprocesses.
In particular,the conversionof the UTP presentin the mixture
to UDP-glucoseand the use of the componentsof the mixture
phosphorylation
to act as cofactorsin the hexokinase-catalyzed
of glucosel5were both uneventful.
F o r t h e s y n t h e s i os f U D P - g l u c o s e( U D P - G l c ) , 4 0 m L o f t h e
mixture of cofactor (6.2 mmol of UTP) was diluted to 200 mL
(pH 8.0). PAN-immobilized UDP-Glc pyrophosphorylase
( U D P G P . E . C . 2 . 7 . 7 . 95, 0 u n i t si n I m L o f g e l ) ,i n o r g a n i cp y r o p h o s p h a t a(sPeP a s eE, . C . 3 . 6 . 1 . 1 , 6u0n i t si n 0 . 5 m L o f g e l ) ,
p h o s p h o g l u c o n r u t(aPsG
e M , E . C . 2 . 1. 5 . 1 ,5 2 u n i t si n I m L o f g e l ) ,
from 6.2 mmol of
a n d g l u c o : c6 - p h o r p h a t (eG - 6 - P ,g e n e r a t e d
b a r i u ms a l tb i t r e l t i n g \ \ r t h D o w e x, 5 0t o r e m o v eb a r i u mi o n ) l s
r i c r e a d d c d I h c r c i l c t i ( ) nr v a sc o n d u c t c du n d e r a r g o n f o r 2 0 h
w i t h p H c o n t r o l l c ad t 7 . 5 . F . n z r m a t iacn a l r s i s ro6f t h e s o l u t i o n
i n d i c a t e di t c o n t a i n e d6 m m o l o i L . D P - G l ca n d l 4 m m o l o f a
m i x t u r e o f o t h e r n u c l e o s i d ter i p h o s p h a t e sA
. f t c r s e p a r a t i o no f
gel,the solutioncouldbe useddirectlyfor
the enzyme-containing
U D P - G l c - r e q u i r i n g d i s a c c h a r i d es y n t h e s i s , roTr i s o l a t e db y
c h r o m a t o g r a p h yu s i n g B i o - R a d P - 2 ( H 2 0 s o l v e n t ) . r 8
The proceduresummarizedin Scheme I providesthe best
method presentlyavailablefor the preparationof GTP, UTP, and
CTP, providedthat the mixture of nucleosidetriphosphatesis
acceptable.We haveexploredan alternativeschemefor generation
hydrolysisof RNA
of this mixturebasedon nuclease.P,-catalyzed
to a mixture of nucleosidentonophosphatcs,
followedby phosphorylationof thesespeciesto triphosphates.reWe havenot found
t h e n u c l e o s i dm
e o n o p h o s p h a tkei n a s e sr e q u i r e df o r t h i s s c h e m e
t o b e e i t h e r e a s i l l p r e p a r e do r e a s i l yh a n d l e d .
The major weaknessof the schemedescribedhere is the present
expenseof commercial PNPase. We note, however,that this
enzymeis readilyavailablefrom f. Coli F20and could in any event
( l l ) T h e e n z y m eP N P a s ef r o m M . L y s o d e i k t i c u w
s a s i m m o b i l i z e da c c o r d i n gt o t h e s t a n d a r dp r o c e d u r e . ?
( 1 2 ) D e t e r m i n e db y P E P a n d P K c o u p l e dw i t h l a c t i cd e h y d r o g e n a s e .
(13) Mononucleotides,
polynucleotides
with therminal3'dinucleotides,
phosphategroups,and double-stranded
polynucleotides
are not substrates
of
PNPase: Godefroy,T. Eur. J. Biochem.1979,14,222-31. Godefroy.T.;
M. Ibid.1970.12.236-49.Chou.J. Y.; Singer.
Cohn,M.; Grunberg-Manago,
M . F . . / . B i o l . C h e m . 1 9 7 0 .2 4 5 . 9 9 5 - 1 0 0 4 .
( 1 4 ) H P L C w a s p e r f o r m e db y u s i n ga W a t e r sR a d i a l - P A KC 1 6c o l u m n
( 5 - m m i . d . ) w i t h - 5m M t e t r a b u t y l a m m o n i u p
mh o s p h a t ien a q u e o u sa c e t o n i t r i l e ( 1 8 %v l v , H 7 . 6 ) a s t h e m o b i l ep h a s e .
( l 5 ) W o n g ,C . - H . ;W h i t e s i d e G
s ,. M . J . A m . C h e m .S o c .1 9 8 1 ,/ 0 J , 4 8 9 0 .
( 1 6 ) D e t e r m i n e de n z y m a t i c a l l yw i t h U D P - g l u c o s ed e h y d r o g e n a saen d
N A D : s e eB e r g m e y e r . a
( 1 7 ) W o n g ,C . - H . ; H a y n i e ,S . L . ; W h i t e s i d e sG, . M . J . O r g .C h e m . 1 9 8 2 .
47.5416-5418.
( l8) Previoup
s r e p a r a t i o nosi U D P - g l u c o s e :M o f f a t , J . G . M e t h o d sE n zymol. 1968,8, 136-42. Kawaguchi,K.; Kawai, H.; Tochikura,T. M ethods
Carbohydr.Chem. 1980,8, 261-9.
( 1 9 ) L e u c h sH
, . J . ; t - e w i sJ, . M . ; R i o s - M e r c a d i l l oV,. M . ; W h i t e s i d e sG. .
M . J . A m . C h e m .S o c ,1 9 7 9 .1 0 1 . 5 8 2 9 - 3 0 .
117
p r o b a b l yb e m a d e i n q u a n t i t y b y r e c o m b i n a n tD N A m e t h o d s .
R e g i s t rN
y o .A T P .5 6 - 6 - 5 -U, sT; P .6 3 - 3 9 - 8G; T P ,8 6 - 0 1 - lC
; T P .6 5 4 7 - 4n
; u c l e a sPe, .- 5 4 5 7 6 - 8 4P- 0
N:P a s e9,0 11 - 1 2 - 4P: E P .I 3 8 - 0 8 - 9P;K ,
9 0 0I - , s 9 - 6U: D P - G l c I. 3 3 - 8 9I-: g l u c o s e5,0 - 9 9 - 7( ;; - 6 - P .5 6 - 7 1 - - s .
SupplementaryMaterial Available: Proceduresfor preparation
of glucose-6-phosphate
by usingthe XTP's preparedhereand for
immobilizationof PNPase( I page). Orderinginformationis given
o n a n y c u r r e n t m a s t h e a dp a g e .
(20) Kimhi, Y.; Littauer. U. Z. Methods En:vmol. 1968. 128. -sl-l-9.
Starting fr,lm 540 g oi the frozen cells,880 units of Pr-Pase were detected.
Alter several steps of purification, 150 units of the enzyme were isolated with
specific activity 6 unit/mg ( I unit will generate I irmol of ATP/min from
poly(A) in a coupled reaction).