AR UP Laboratories 500 Chipeta Way
advertisement

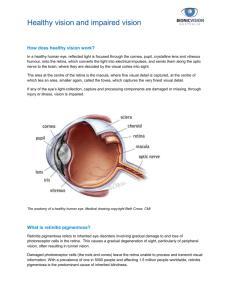
*** Example Report *** ARUP Laboratories 500 Chipeta Way- Salt Lake City, UT 84108 (800) 522-2787 - www.aruplab.com Sherrie L. Perkins, MD, Laboratory Director Procedure Retinitis Pigmentosa Del/Dup Specimen Retinitis Pigmentosa Del/Dup Interp EER Retinitis Pigmentosa Del/Dup Result Whole Blood See Note f See Note Patient Age/Gender: 5 years Male Printed: 25-Sep-12 13:32:49 Units Ref Interval Access ion Collec ted Rec e ived 12 - 262 - 100106 18 - Sep- 12 11:29:00 12-262-100106 18 - Sep- 12 11 : 29 : 00 12 - 262 - 100106 18 - Sep- 12 11 : 29 : 00 18 - Sep- 12 11 : 31:00 18 - Sep- 12 11 : 31 : 00 18 - Sep-1 2 11 : 31 : 00 Verified 21 - Sep- 12 13 : 46:29 21 - Sep-12 13:46:29 21 - Sep- 12 13:46 : 29 * = Abnormal, # = Corrected, C = Critical, f = Footnote, H = High, L = Low, t = Interpretive Text,@= Reference Lab Chart ID: 4102401 Page 1 of3 *** Example Report *** ARUP Laboratories 500 Chipeta Way- Salt Lake City, UT 84108 (800) 522-2787 - www.aruplab.com Sherrie L. Perkins, MD, Laboratory Director 18-Sep-12 11 : 29:00 Patient Age/Gender: 5 years Male Printed: 25-Sep-12 13 :32:49 Retinitis Pigmentosa Del/Dup Interp : Section 79-1 of New York State Civil Rights Law requires informed consent be obtained from all patients (or their legal guardians) prior to pursuing any diagnostic genetic testing or testing to assess carrier status. These forms must be kept on file by the ordering physician . Biochemical and DNA testing patient consent forms can be accessed from ARUP ' s web site : www . aruplab . com . Reason for referral : Patient symptoms listed here . Result: No pathogenic mutations were detected. Interpretation : No pathogenic deletions or duplications were detected using a custom designed Comparative Genomic Hybridization (CGH) array covering 53 genes associated with retinitis pigmentosa (RP)/Leber congenital amaurosis (LCA) . Recommendations : This test does not detect all mutations associated with RP/LCA. Consideration should be given to testing for sequence variations of the 53 genes associated with RP/LCA (Retinitis Pigmentosa/Leber Congenital Amaurosis Sequencing , 53 Genes ; ARUP test code 2007091). Medical screening and management should rely on clinical findings and family history . Genetic consultation is recommended. Reference Genome : Human genome build 19 (Hg 19) . This result has been reviewed and approved by Pinar Bayrak- Toydemir , M.D. , Ph.D. Background Information for Retinitis Pigmentosa (RP) Sequencing and Dele tion/Dupli cat ion: Characteristics : Retinitis pigmentosa (RP) is a collection of inherited irregularities that can affect the photoreceptors (rods and cones) and retinal pigment epithelium leading to continuous visual deterioration. Patients with RP typically experience "night blindness" first, followed by a narrowing of the peripheral vision field. Ultimately the disease progresses t o a complete loss of central vision . Symptoms may occur in childhood but severe vision problems do not usually develop until early adulthood. Leber Congenital Amaurosis (LCA) is a severe retinal dystrophy usually observed in the first year of life . Poor vision is o ften accompanied by keratoconus , nystagmus, and photophobia . In later childhood patients with LCA may develop a pigmentary retinopathy , similar to that seen in RP . Incidence : RP : 1 in 3,500 - 4 , 000 LCA : 1 in 33 , 000 - 50,000 Inheritance : Variable : RP - autosomal dominant , autosomal recessive or X- linked recessive. LCA - autosomal recessive or rarely autosomal dominant Penetrance : Var iable; dependent on the gene involved . Cause : Pathogenic mutations in genes associated with the development and function of the retina . Clinical Sensitivity: Unknown . Genes Tested : RP: PRPF3, RHO, RDS, RP9, RPl, ROMl , NRL, PRPFS , CA4, FSCN2 , PRPF31, GUCAlB, SEMA4A, TOPORS, BESTl, KLHL7 , SNRNP200, ABCA4 , CERKL , CNGAl, CNGBl , EYS , MERTK , NR2E3, PDE6A, PDE6B , PROMl, RGR , SAG , TTCS, USH2A, RP2 , PRCD, RPGR , DHDDS, RLBPl, IDH3B, C20RF71 , CDHRl LCA: CEP290 , LCA5, RD3 , RDH12 , GUCY2D, RPGRIPl , SPATA7 Both RP and LCA : TULPl, LRAT , AIPLl, CRBl, CRX, IMPDHl, RPE65 Methodology : Deletion/duplication analysis by the tiled custom designed Comparative Genomic Hybridization (CGH) array. Analytical Sensitivity : >96 % Clinical Sensitivity : RP - unknown; LCA - 40 %- 50 % Limitations: Single base pair substitutions , small deletion/duplications , regulatory region mutations, and deep intronic mutations will not be detected. Deletion/duplications breakpoints will not be determined . Mutations in genes not tested for by this assay will not be detected . Copy number variants smaller than one kb will not be detected by deletion/duplication analysis . 18 - Sep - 12 11:29:00 Retinitis Pigmentosa Del/Dup Interp : *=Abnormal,#= Corrected, C = Critical, f= Footnote, H = High, L =Low, t =Interpretive Text,@= Reference Lab ChartiD: 4102401 Page 2 of3 ARUP Laboratories 500 Chipeta Way- Salt Lake City, UT 84108 (800) 522-2787 - www.aruplab.com Sherrie L. Perkins, MD, Laboratory Director *** Example Report *** Patient Age/Gender: 5 years Male Printed: 25-Sep-1 2 13 :32:49 INTERPRETIVE INFORMATION : Retinitis Pigmentosa Del/Dup , 53 Genes The performance characteristics of this test were validated by ARUP Laboratories . The U. S. Food and Drug Administration (FDA) has not approved or cleared this test . However , FDA approval or clearance is currently not required for clinical use of this test . The results are not intended to be used as the sole means for clinical diagnosis or patient management decisions . ARUP is authorized under Clinical Laboratory Improvement Amendments (CLIA) and by all states to perform high - complexity testing . Counseling and informed consent are recommended for genetic testing . available online at www . aruplab . com . Consent forms are * = Abnormal,#= Corrected, C = Critical, f =Footnote, H = High, L = Low, t = Interpretive Text,@= Reference Lab Chart ID: 4102401 Page 3 of3