2. Beta Decay Type of Radioactive Decay
advertisement
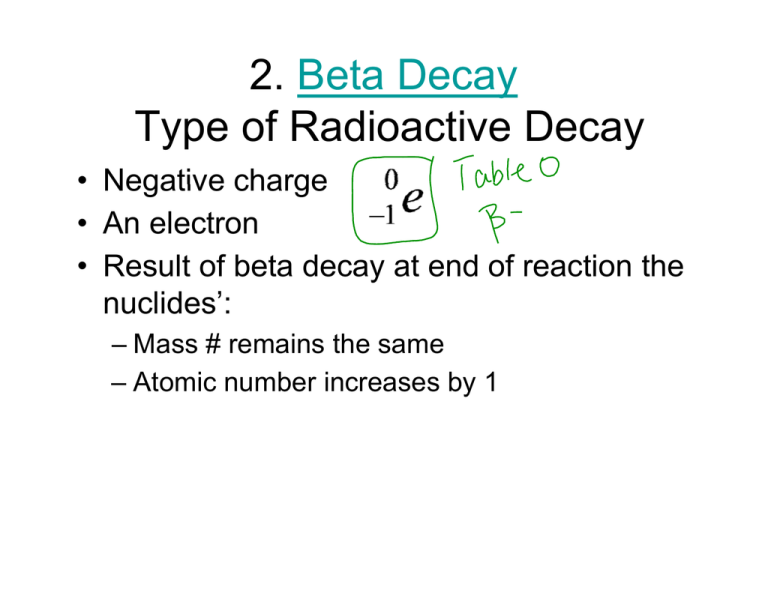
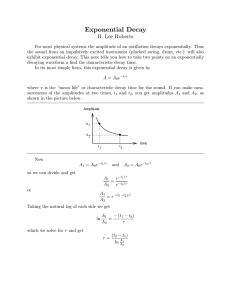
2. Beta Decay Type of Radioactive Decay • Negative charge • An electron • Result att end R lt off beta b t decay d d off reaction ti the th nuclides’: – Mass M # remains i the h same – Atomic number increases by 1 • http://www http://www.ndt ndted.org/EducationResources/HighSchool/R adiography/Graphics/Flash/transmut swf adiography/Graphics/Flash/transmut.swf 3. Positron emission Type of Radioactive Decay • + charge g • Has mass of electron • Result of positron emission at end of reaction the nuclides’: – Atomic number decreases by 1 – Mass stays the same • Positron emission converts a proton to a neutron 4. Gamma Radiation Type of Radioactive Decay • High energy waves emitted from a nucleus as it changes from the excited state to the ground state state. • No Change in the Nucleus! Deflection of Decay Particles • Opposite charges attract each other other. • Like charges repel each other. • http://www http://www.ndt ndted.org/EducationResources/HighSchool/R adiography/Graphics/Flash/transmut swf adiography/Graphics/Flash/transmut.swf • http://www http://www.mhhe.com/physsci/chemistry/e mhhe com/physsci/chemistry/e ssentialchemistry/flash/radioa7.swf • Nuclear decay Summary of the most common forms of radiation Particle Mass Charge g Symbol ym Penetrating g power Alpha 4 amu +2 Low Beta 0 amu -1 Moderate Positron 0 amu +1 Moderate G Gamma 0 amu N None hi h high Table O Half Life= time it takes for ½ the atoms in the radioactive sample to decay into a different substance. Half-life Concept Half-Life • Decay of 20.0 mg of 15O. What remains after 3 half-lives? half lives? After 5 half-lives? half lives? • See Table N Selected Radioisotopes • To find the amount of a sample remaining after a certain amount of days: • 1. Find the half-life on Table N. • 2. Make a table with 2 columns: time and mass • 3. Begin your table with zero time. How much of a 100 gram sample of iodine131 will ill remain i after ft 24 d days? ? TIME MASS 0 days 100 g 8 days 50 g 16 days 25 g 24 days 12.5 g Phosphorous-32 has a half life of 14.3 days. Determine how many half-lives elapse during 57.2 days. How many milligrams remain if you start with 4.0mg? Time Mass The half life of radon-22 is 3.824 days. Aft what After h t time ti will ill one-fourth f th of f a given i amountt of f radon d remain? Time Mass A sample contains 16 mg of polonium-218. After 12 minutes, the sample will contain 1.0 mg of polonium-218. What is the half life of polonium-218? Time m Mass The half life of I-123 is 13 hr. hr How much of a 64 mg sample of I-123 is left after 39 hours?