Unit Prefixes & Multipliers: Chemistry Worksheet
advertisement

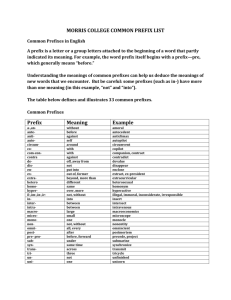
Think Ahead Section 1.6 Chemistry 150 Unit Prefixes/Prefix Multipliers Unit Prefixes/Prefix Multipliers You can get help with this work from the following sources: 1. Visit your instructor during office hours 2. Go to the Native American Center 3. Use your textbook (Table 1.2 and back page of textbook, “SI Unit Prefixes”). 4. Use the following resources on the Internet: • http://www.education.com/study-help/article/physics-help-prefixmultipliers/ • http://www.youtube.com/watch?v=57JuE6Ya46Y Concept 1: Know how to convert a prefix multiplier to its numerical value. Prefix multipliers are capital and small case letters that represent specific numbers that are powers of ten. The prefix multipliers that are most commonly used in chemistry are pico (p), nano (n), micro (µ), milli, (m) and kilo (k) and mega (M). Note that these letters are just numbers: kilo = 1000 (or 103). They are not units per se, rather they are (often) part of a unit. For example, for a kilometer (km), the unit is meter and the kilo means 1000; therefore a km is 1000 meters. Problem: Give the number value corresponding to each of the following unit prefixes: G= µ= f= M= n= c= m= P= Concept 2: Know how to use prefix multipliers in dimensional analysis problems. It is important that you develop an intuitive sense for what the numerical values of the unit prefixes mean. For example, when looking at Table 1.2, you see that µ = 10-6. So then why do we say that there are 106 micrograms in a gram? This is because there are 10-6 grams in one pictogram. Therefore, both of the conversion factors below are valid and can be used in dimensional analysis problems: 106 pg g 10-6 g pg Think Ahead Section 1.6 Chemistry 150 Unit Prefixes/Prefix Multipliers Problem: How many picograms (pg) are in 13.4 kg of lead? ***Place for LG to do calculator part Concept 3: Know how to enter unit prefix values into your calculator. It is often quicker to enter the numerical value of a unit prefix into your calculator rather than converting from one unit prefix to another. You can use your EE button to enter the value of a unit prefix; for example, for k you would enter EE3 or for m you would enter EE-6. Problem: Note: this problem requires you to know about Avogadro’s number, which is covered in Tro Section 2.9. How many atoms are in 55 millimoles (mmol) of copper? ***Place for LG to do calculator part More Practice Problems: p 39, 75, 76. (Answers are found in A-16.)