Advance Access published March 12, 2004
Journal of Experimental Botany, Page 1 of 9
DOI: 10.1093/jxb/erh094
RESEARCH PAPER
Regulation of sulphate assimilation by glutathione in
poplars (Populus tremula3P. alba) of wild type and
overexpressing g-glutamylcysteine synthetase in the
cytosol
1
Albert-Ludwigs-University of Freiburg, Institute of Forest Botany and Tree Physiology, Georges-KoÈhler-Allee
053, D-79110 Freiburg, Germany
2
Heidelberger Institute of Plant Sciences (HIP), Im Neuenheimer Feld 360, D-69120 Heidelberg, Germany
Received 17 October 2003; Accepted 26 December 2003
Abstract
Glutathione (GSH) is the major low molecular weight
thiol in plants with different functions in stress
defence and the transport and storage of sulphur. Its
synthesis is dependent on the supply of its constituent amino acids cysteine, glutamate, and glycine.
GSH is a feedback inhibitor of the sulphate assimilation pathway, the primary source of cysteine synthesis. Sulphate assimilation has been analysed in
transgenic poplars (Populus tremula3P. alba) overexpressing g-glutamylcysteine synthetase, the key
enzyme of GSH synthesis, and the results compared
with the effects of exogenously added GSH.
Although foliar GSH levels were 3±4-fold increased in
the transgenic plants, the activities of enzymes of
sulphate assimilation, namely ATP sulphurylase, adenosine 5¢-phosphosulphate reductase (APR), sulphite
reductase, serine acetyltransferase, and O-acetylserine (thiol)lyase were not affected in three transgenic
lines compared with the wild type. Also the mRNA
levels of these enzymes were not altered by the
increased GSH levels. By contrast, an increase in
GSH content due to exogenously supplied GSH
resulted in a strong reduction in APR activity and
mRNA accumulation. This feedback regulation was
reverted by simultaneous addition of O-acetylserine
(OAS). However, OAS measurements revealed that
OAS cannot be the only signal responsible for the
lack of feedback regulation of APR by GSH in the
transgenic poplars.
Key words: Adenosine 5¢-phosphosulphate reductase,
cysteine synthesis, glutathione, poplar, sulphate assimilation.
Introduction
The majority of the essential element, sulphur, in living
organisms is in the reduced form of organic thiols, such as
the amino acids cysteine and methionine. Plants, yeast, and
bacteria are primary producers of these organic thiols since
they are able to reduce sulphate, the major form of sulphur
in nature, to sulphide and incorporate it in an activated
amino acid acceptor to build cysteine or homocysteine
(Brunold, 1990; Leustek et al., 2000). The ®rst enzyme
involved in this pathway of assimilatory sulphate reduction
(Fig. 1) is ATP sulphurylase (ATPS) which catalyses the
activation of sulphate by ATP to adenosine 5¢-phosphosulphate (APS). In plants and the majority of bacteria, APS
is reduced to sulphite by APS reductase (APR) (Suter et al.,
2000; Kopriva et al., 2002), whereas in fungi and some
enteric bacteria APS must be phosphorylated to phosphoadenosine 5¢-phosphosulphate and only this compound can
be reduced to sulphite (Jones-Mortimer, 1973; Marzluf,
1996). Sulphite is further reduced by sulphite reductase
* To whom the correspondence should be addressed. Fax: +49 761 2038302. E-mail: Stanislav.Kopriva@ctp.uni-freiburg.de
Abbreviations: APR, adenosine 5¢-phosphosulphate reductase; APS, adenosine 5¢-phosphosulphate; ATPS, ATP sulphurylase; GSH, glutathione; GSHS,
glutathione synthetase; g-ECS, g-glutamylcysteine synthetase; OAS, O-acetylserine; OASTL, O-acetylserine (thiol)lyase; SAT, serine acetyltransferase; SIR,
sulphite reductase.
Journal of Experimental Botany, ã Society for Experimental Biology 2004; all rights reserved
Downloaded from http://jxb.oxfordjournals.org/ at Pennsylvania State University on February 20, 2013
Tanja Hartmann1, Petra HoÈnicke1, Markus Wirtz2, RuÈdiger Hell2, Heinz Rennenberg1 and
Stanislav Kopriva1,*
2 of 9 Hartmann et al.
(SIR). The resulting sulphide is used for cysteine
biosynthesis by O-acetylserine (thiol)lyase (OASTL)
using O-acetylserine, which is synthesized from serine
by serine acetyltransferase (SAT). Cysteine can be used
directly for protein synthesis, further metabolized to
methionine, or serve as a sulphur donor for the synthesis
of coenzymes (thiamine, CoenzymeA, molybdopterine)
and glutathione (g-glutamyl-cysteinyl-glycine, GSH).
GSH is the most abundant low molecular weight thiol in
plants and plays an important role in the defence against
various biotic and abiotic stress conditions and in redox
buffering of the cell (Noctor et al., 1998a; May et al.,
1998). Moreover, GSH is, beside S-methylmethionine, the
main storage and transport form of reduced sulphur and is
involved in the regulation of sulphate assimilation (Foyer
and Rennenberg, 2000).
GSH is synthesized in the cytosol and in plastids of plant
cells from its constituent amino acids via the consecutive
action of g-glutamylcysteine synthetase (g-ECS), synthesizing g-glutamylcysteine (g-EC) from glutamate and
cysteine, and glutathione synthetase (GSHS), adding
glycine to the C-terminal end of g-EC (Foyer and
Rennenberg, 2000). At the cellular level, glutathione
biosynthesis is limited by the availability of cysteine
(Strohm et al., 1995; Noctor et al., 1996), which itself is
dependent on the supply of both reduced sulphur and
carbon precursor (Blaszczyk et al., 1999; Harms et al.,
2000). Furthermore, GSH synthesis is regulated by the
activity of g-ECS which, in vitro, is frequently feedbackinhibited by glutathione (Hell and Bergmann, 1990;
Schneider and Bergmann, 1995; Strohm et al., 1995).
Indeed, overexpression of g-ECS, but not GSHS, in hybrid
poplars (Populus tremula3P.alba) led to an approximately
3-fold increase in foliar GSH concentrations compared
with wild-type plants, but did not affect cysteine levels
Materials and methods
Plant material
Experiments were performed with young wild-type and transformed
hybrid poplars (Populus tremula3P.alba, INRA clone 717 1B4)
overexpressing the bacterial gene for g-ECS in the cytosol (lines
ggs5, ggs11, ggs28: Noctor et al., 1996; Arisi et al., 1997). The
plants were micropropagated in vitro as previously described
(Strohm et al., 1995) and transferred to pots with moistened balls
(2±6 mm in diameter) of burned clay (Leca Ton, Leca, Lamstedt,
Germany). They were further grown in the greenhouse with a 16/8 h
Downloaded from http://jxb.oxfordjournals.org/ at Pennsylvania State University on February 20, 2013
Fig. 1. Scheme of sulphate assimilation and glutathione synthesis in
plants. Feedback inhibition of ATP sulphurylase and APS reductase
by GSH and the inhibition of g-glutamylcysteine synthetase by
buthionine sulphoximine (BSO) are indicated.
(Noctor et al., 1996; Arisi et al., 1997). The increased GSH
synthesis is independent from localization of the overexpressed protein in the chloroplast or the cytosol; all these
transgenic plants share similar basic metabolism, biochemistry, and physiology with wild-type poplars (Arisi
et al., 1997; Noctor et al., 1998b).
Key points of regulation of assimilatory sulphate
reduction by GSH are the enzymes ATPS and APR. In
experiments with Brassica napus and Arabidopsis thaliana
low GSH levels in the phloem due to sulphur deprivation
correlated with increased ATPS activities and mRNA
levels (Lappartient and Touraine, 1996; Lappartient et al.,
1999). Supplying external cysteine or GSH inhibited
ATPS activity and mRNA accumulation. Buthionine
sulphoximine (BSO), an inhibitor of g-ECS (Fig. 1),
reversed the inhibitory effect of cysteine on ATPS leading
to the conclusion that GSH and not cysteine is responsible
for the regulation of ATPS in response to sulphur
availability. On the other hand, Bolchi et al. (1999)
showed that cysteine was able to down-regulate the ATPS
transcript level when supplied to sulphur-de®cient Zea
mays seedlings under conditions when GSH synthesis was
blocked with BSO. However, different authors found APR
more susceptible to a range of regulatory signals than
ATPS (Lee and Leustek, 1999; Koprivova et al., 2000;
Westerman et al., 2001). Signi®cantly, H2S fumigation of
Brassica oleracea led to an increase in cysteine and GSH
levels in the shoot, accompanied by a decrease in APR but
not in ATPS activity (Westerman et al., 2001). Indeed,
externally applied cysteine and GSH to Arabidopsis root
cultures decreased APR mRNA, protein, and activity while
ATP sulphurylase was much less affected by the treatments. Flux control analysis revealed that APR possess
more than 50% control over sulphate assimilation
(Vauclare et al., 2002). When BSO was added simultaneously, cysteine had no effect on APR activity, whereas
GSH decreased APR mRNA, indicating that also in
Arabidopsis GSH and not cysteine is the regulatory
substance of the sulphate assimilation pathway (Vauclare
et al., 2002).
Investigations with transgenic poplar lines overexpressing g-ECS in the cytosol, aimed at comparing the effects
of increased internal GSH synthesis and externally
supplied GSH on sulphate assimilation, are reported here.
Regulation of sulphate assimilation in poplar 3 of 9
light/dark regime in hydroponic culture (0.3 mM Ca(NO3)2, 0.4 mM
KNO3, 0.025 mM CaCl2, 0.3 mM MgSO4, 0.05 mM KCl, 0.26 mM
NaH2PO4, 0.1 mM FeSO4, 0.1 mM Na-EDTA, 2 mM MnSO4, 10 mM
H3BO3, 0.2 mM CuSO4, 0.2 mM ZnSO4, 0.2 mM Na2MoO4, and 40
nM CoSO4) for 6±10 weeks. For feeding of intact plants the nutrient
solution also contained 1 mM glutathione pH 6.0, 1 mM buthionine
sulphoximine (BSO), 1 mM O-acetylserine (OAS) or 1 mM GSH
and 1 mM OAS, simultaneously.
Enzyme assays
Activities of ATP sulphurylase, APS reductase, sulphite reductase,
and O-acetylserine(thiol)lyase were measured in the tenth leaf from
the apex in transgenic and wild-type poplars. For determination of
serine acetyltransferase leaves 9±11 from the apex were pooled. All
leaf samples were taken between 08.00 h and 10.00 h. Extraction of
enzymes, enzyme assays, and protein determination were carried out
as described previously (Hartmann et al., 2000).
ATPS activity was measured using the luciferin±luciferase system
to quantify the ATP produced (Schmutz and Brunold, 1982). The
reaction vial contained 20 ml 0.1 mM APS, 100 ml luciferin±
luciferase mixture, 5 ml 1:20 diluted extract, and 100 ml 165 mM PPi.
The initial rate of ATP production was followed for 30 s with a
luminometer (Lumat LB 9501, Berthold, Germany).
APR activity in extracts was measured as the production of
[35S]sulphite, assayed as acid volatile radioactivity formed in the
presence of 75 mM [35S]APS (15±30 kBq mmol±1) and 4 mM DTE
(Brunold and Suter, 1990).
SIR activity was measured by coupling the production of sulphide
with synthesis of cysteine by OASTL present in the extracts in
excess (von Arb and Brunold, 1983). The enzyme assay contained
0.18 M Tricine±NaOH pH 7.4, 0.7 mM methyl viologene, 6 mM
OAS pH 6.0, and 6 mM sodium dithionite (dissolved in 150 mM
NaHCO3) in a volume of 1 ml. The cysteine formed was derivatized
with monobromobimane, detected, and quanti®ed by HPLC
analysis.
OASTL activity was measured in an assay mixture containing
0.2 M TRIS-HCl pH 7.5, 10 mM DTE, 8 mM OAS, and 8 mM Na2S.
After 10 min at 30 °C the cysteine produced was determined
photometrically at 546 nm as a red ninhydrin complex.
Measurement of SAT activity was performed by a coupled assay,
using the excess OASTL present in the protein extract instead of
addition of puri®ed enzyme (Nakamura et al., 1987). The reaction
mixture (200 ml) contained 140 ml leaf extract (corresponding to c.
1 mg protein), 75 mM TRIS pH 8.0, 0.5 mM DTT, 10 mM serine,
2.5 mM Na2S, and 1 mM acetyl-CoA. Reactions were started by the
addition of acetyl-CoA and the samples were incubated at 30 °C for
cDNA cloning
The cDNA clones for ATPS (GenBank accession number
AY353090), APR (AY353089), and cytosolic isoforms of OASTL
(AY353092) and SAT (AY353091), were obtained by screening a
cDNA library from poplar leaf RNA, constructed in Lambda ZAP
phages with the ZAP-cDNA synthesis kit (Stratagene, Amsterdam,
The Netherlands) with corresponding cDNA clones from
Arabidopsis. SIR (AY353094) and tubulin (AY353093) cDNAs
were obtained by RT-PCR ampli®cation of total RNA from poplar
leaves with oligonucleotide primers derived from conserved
domains. The ATPS cDNA probe, corresponding to accession
number U05218, was derived from Arabidopsis total RNA by RTPCR ampli®cation. The identity of the cDNA fragments was veri®ed
by sequencing. Cytosolic isoforms of SAT and OASTL were chosen
for the expression analysis because, in these transgenic poplars, the
cytosolic GSH pool was increased (Hartmann et al., 2003).
RNA blot analysis
The tenth leaves from the top of poplar plants were pulverized in a
mortar and pestle in liquid nitrogen. Total RNA was isolated from
®ve poplars per treatment with the RNeasyâ extraction kit (Plant
Mini Kit, Quiagen, Hilden, Germany) according to the manufacturer's instruction. Electrophoresis of RNA was performed on 1%
formaldehyde±agarose gels at 120 V. RNA was transferred onto
Hybond-N and hybridized with 32P-labelled cDNA probes. The
membranes were washed three times at different concentrations of
SSC in 0.1% SDS for 20 min, the ®nal washing step being 13 SSC,
0.1% SDS at 65 °C for 1 h, and exposed to an X-ray ®lm (Kodak
BioMax MS, Integra Biosciences, Fernwald, Germany) for 1±3 d.
The autoradiograms were quanti®ed with a densitometer GS-670
(BioRad, Munich, Germany) using the software Molecular Analyst
(BioRad) and Adobe PhotoshopTM.
Determination of O-acetylserine (OAS)
100 mg tissue material was ground in liquid nitrogen to a ®ne
powder and extracted with 1 ml 0.1 M HCl for 15 min at 4 °C while
shaking. Homogenates were centrifuged twice at 15 400 g, 4 °C for 5
min. The resulting supernatants were used for further analysis.
Determination of OAS was based upon derivatization with the
¯uorescence dye AccQ-Tag (Waters, USA). An appropriate volume
of supernatant (5±20 ml) was derivatized according to the manufacturer (Manual WAT052874TP). The resulting OAS derivative was
separated form other amino acid derivatives by reverse phase HPLC
as described in Rolletschek et al. (2002), with the exception that the
pH of buffer A was optimized to pH 6.3. Data acquisition and
processing was performed with Millenium32 software (Waters,
USA). Recovery rates for OAS were higher than 90% in all tested
tissues as determined by spiking of samples with internal standards.
All samples were analysed in triplicate.
Statistical analysis
Statistical analysis was performed using the Tukey Test (P <0.05) or
T-test (P <0.05) with SPSS for Windows, Release 9.0.
Results
Effect of enhanced GSH synthesis on sulphate
assimilation in transgenic poplar
The effect of enhanced GSH synthesis on the sulphate
assimilation pathway was investigated using transgenic
Downloaded from http://jxb.oxfordjournals.org/ at Pennsylvania State University on February 20, 2013
Analysis of low molecular weight thiols
Low molecular weight thiols were analysed in leaf and root extracts
as described before (Herschbach et al., 2000). About 30 mg frozen
powder was transferred into precooled (4 °C) vials containing 750 ml
0.1 M HCl and 50 mg insoluble PVPP and were subsequently
centrifuged for 10 min at 12 000 g and 4 °C. 120 ml aliquots of the
supernatants were adjusted to pH 8.360.2 with 180 ml 200 mM
CHES pH 9.3. Oxidized thiols were reduced by adding 30 ml 15 mM
DTT for 60 min. Derivatization was performed with 45 ml 30 mM
monobromobimane (ThiolyteâMB, Calbiochem, Bad Soden,
Germany) for 15 min at room temperature and subsequently the
bimane derivatives were stabilized with 240 ml 10% acetic acid
(v/v). Bimane derivatives were separated and quanti®ed by ¯uorescence detection as described by Schupp and Rennenberg (1988).
Peaks were identi®ed and quanti®ed using a standard solution
containing 0.1 mM Cys, 0.1 mM g-EC, and 1 mM GSH in 0.1 M
HCl.
20 min. The cysteine produced was detected and quanti®ed by HPLC
analysis as described above.
4 of 9 Hartmann et al.
poplars overexpressing g-glutamylcysteine synthetase in
the cytosol (Noctor et al., 1996; Arisi et al., 1997).
Enzymatic activities and mRNA levels of ATPS, APR,
SIR, OASTL, and SAT were measured in the tenth leaves
of transgenic lines ggs5, ggs11, and ggs28 (Fig. 2). All
investigated plant lines have about 2±3-times higher foliar
GSH concentration than the wild-type plants, whereas
cysteine levels are only slightly enhanced (Arisi et al.,
1997). As shown in Fig. 2, the higher thiol contents did not
affect the enzyme activities of APR, SIR, OASTL, or SAT;
ATPS was increased in two transgenic lines. Steady-state
mRNA levels for APR and SIR were not affected in any
Effect of exogenously applied GSH or BSO on
sulphate assimilation in wild-type poplar
Since the well-described feedback inhibition of ATPS and
APR by an increased concentration of GSH was not
observed in the experiments with transgenic poplars (Fig. 2,
3), it was tested whether an increased GSH content by
exogenously applied GSH or its decrease by inhibition of
g-ECS with BSO (Grif®th, 1982) affects the mRNA level
and activity of APR. APR was chosen for these experiments since Vauclare et al. (2002) showed that APR
possess a more than 50% control over the ¯ux through the
Fig. 2. Transcript levels and activities of enzymes involved in
assimilatory sulphate reduction in leaves of poplar trees
overexpressing g-ECS in the cytosol. Total RNA (5 mg) was isolated
from the tenth leaf from the apex of transgenic and wild-type poplar
plants, separated on 1% agarose in the presence of formaldehyde,
blotted onto Hybond-N nylon membrane, and hybridized with 32Plabelled cDNA fragments of ATP sulphurylase (A), APS reductase
(C), sulphite reductase (E), O-acetylserine(thiol)lyase (G), and serine
acetyltransferase (I). The transcript levels were normalized to the
expression of b-tubulin; the mRNA levels in wild-type plants were set
to 1. Data shown are means 6SD of at least three plants. In vitro
activities of ATP sulphurylase (B), APS reductase (D), sulphite
reductase
(F),
O-acetylserine(thiol)lyase
(H),
and
serine
acetyltransferase (J) were measured in extracts of the tenth leaves
from apex of transgenic and wild-type poplars as described in
Materials and methods. Data shown are means 6SD of ®ve (B), six
(J) or seven (D, F, H) extracts from independent poplar trees measured
in triplicate.
Fig. 3. Thiol contents and APR transcript levels in ®ne roots of poplar
trees overexpressing g-ECS in the cytosol. (A) Contents of cysteine
(white bars, right axis) and GSH (black bars, left axis) were measured
¯uorometrically following reduction by DTT, derivatization with
monobromobimane, and separation by reversed-phase HPLC. Mean
values 6SD of ®ve independent plants are presented. The asterisks
show signi®cant differences to the wild-type plants at P <0.05. (B)
APR transcript levels. Total RNA (5 mg) isolated from the roots, was
separated on 1% agarose in the presence of formaldehyde, blotted
onto Hybond-N nylon membrane, and hybridized with 32P-labelled
APR cDNA fragment. The transcript levels were normalized to the
expression of b-tubulin; the mRNA levels in wild-type plants was set
to 1. Data shown are means 6SD of three plants.
Downloaded from http://jxb.oxfordjournals.org/ at Pennsylvania State University on February 20, 2013
line. The mRNA level of the cytosolic isoform of OASTL
was reduced in line ggs5, but comparable to the wild type
in the other lines, whereas the transcript level of cytosolic
SAT slightly increased in ggs11 and ggs28, but were not
affected in ggs5. The increased activity of ATPS was not
accompanied by a corresponding increase in mRNA levels.
In addition, roots of the transgenic lines ggs5 and ggs28
contained GSH concentrations 2-fold higher than wildtype roots (Fig. 3A). Cysteine and g-EC contents did not
differ between the transgenic and wild-type roots. As
already shown for leaves, APR mRNA was accumulated in
roots to the same level in the wild type and both transgenic
lines and was not affected by the increased GSH concentration (Fig. 3B).
Regulation of sulphate assimilation in poplar 5 of 9
sulphate assimilation pathway. Preliminary experiments
revealed that, in the leaves, the effects of GSH and BSO
feeding via the roots are much smaller than in the roots,
therefore further experiments were performed with the
roots. Figure 4 illustrates the effects of the addition of 1
mM GSH or 1 mM BSO to the roots of wild-type poplars
for 3 d. Feeding of exogenous GSH increased the GSH
contents in the roots approximately 2-fold and also led to a
5-fold increase in cysteine contents (Fig. 4A). This resulted
in a 50% decrease in APR activity and mRNA accumulation (Fig. 4B, C, D). The addition of BSO strongly
decreased GSH levels in the roots by about 95% and also
led to about a 2-fold increase in cysteine. The decrease in
GSH content resulted in a big increase in APR transcript
abundance (Fig. 4C, D). The changes in mRNA levels
were accompanied by an approximately 4-fold increase in
APR activity (Fig. 4B). These results also show, that in
poplars, APR is regulated by GSH at the transcriptional
level.
OAS attenuates the feedback inhibition of APR by
GSH
Discussion
Poplars overexpressing bacterial genes for g-ECS and
GSHS in the cytosol or in the chloroplast are the best
characterized plants with manipulated GSH content. These
plants have contributed signi®cantly to the understanding
of the regulation of GSH synthesis by its constituent amino
acids (Strohm et al., 1995; Noctor et al., 1996, 1997,
1998b). Due to the increased GSH concentration in the
Downloaded from http://jxb.oxfordjournals.org/ at Pennsylvania State University on February 20, 2013
Fig. 4. Effect of glutathione or BSO on thiol contents and APR
transcript level and activity in poplar roots. Poplar plants were treated
by addition of 1 mM GSH or 1 mM BSO to the normal nutrition
solution for 3 d. (A) Contents of cysteine (white bars) and GSH (black
bars) in roots were measured ¯uorometrically following reduction by
DTT, derivatization with monobromobimane and separation by
reversed-phase HPLC. Mean values 6SD of ®ve independent plants
are presented. Bars with different letters indicate values signi®cantly
different with P <0.05. (B) APR activity was measured in extracts
from ®ne roots. Mean values 6SD of ®ve plants are presented. (C)
Total RNA (5 mg) isolated from roots of four to ®ve plants, was
separated on 1% agarose in the presence of formaldehyde, blotted
onto Hybond-N nylon membrane, and hybridized with 32P-labelled
APR cDNA fragments. Relative APR mRNA levels were measured
densitometrically and normalized to rRNA. A value of 1.0 represents
the mRNA level in wild-type plants. (D) Representative northern blot
of APR. Ethidium-bromide-stained ribosomal RNA (rRNA) is shown
as a control of loading and RNA intactness.
The simplest explanation for the contradictory results
presented in Figs 3 and 4 is that, in the transgenic plants,
feedback regulation of APR by GSH was overcome by
another signal. O-acetylserine (OAS), a substrate for Cys
synthesis, is known to regulate sulphate uptake and APR
positively and, thus, would be a good candidate for such a
signal. Therefore, OAS was measured in leaves and roots
of wild-type and transgenic poplars and in poplars treated
with GSH. OAS levels were increased 2-fold in the leaves
of the transgenic line ggs5 and slightly, but not signi®cantly, increased in lines ggs11 and ggs28 compared with
wild-type levels (Table 1). Roots of the line ggs5 contained
lower OAS levels compared with the wild type, whereas in
the other two lines OAS contents were not signi®cantly
affected.
In addition, wild-type poplar roots were treated with
GSH and OAS alone or in combination for 72 h. These
treatments doubled the concentration of GSH and elevated
OAS levels 5±8-fold (Fig. 5A, B). Cysteine contents were
increased 6±7-fold by all three treatments. APR activity
was not signi®cantly altered by the different treatments
(Fig. 5C). As expected, the APR mRNA level was
increased 4-fold by OAS treatment and decreased by
50% upon addition of GSH (Fig. 5D). Simultaneous
addition of OAS and GSH resulted in APR mRNA levels
between those of controls and treatments with OAS alone.
However, since the roots of transgenic poplars have no
feedback inhibition of APR despite high GSH concentration and constant OAS and OAS levels were not affected
by the GSH treatment, OAS cannot be the only signal to
overcome the feedback inhibition of APR by GSH.
6 of 9 Hartmann et al.
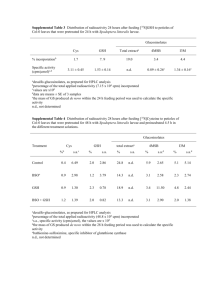
Table 1. OAS contents in wild-type poplars and in three
transgenic lines overexpressing g-ECS in the cytosol
OAS contents shown represent means 6SD of 3±4 separate
extractions of the tenth leaves from the apex and of ®ne roots. An
asterisk shows signi®cant differences from the wild-type plants at
P <0.05.
Wild type
ggs5
ggs11
ggs28
OAS in leaves
(nmol g±1 FW)
OAS in roots
(nmol g±1 FW)
2.4360,82
6.5460.77*
3.3660.67
3.7760.92
4,5760.94
2.1060.75*
4.3961.09
8.4863.33
Downloaded from http://jxb.oxfordjournals.org/ at Pennsylvania State University on February 20, 2013
plants overexpressing g-ECS (Noctor et al., 1996, 1997;
Arisi et al., 1997) an additional sink for reduced sulphur
was formed. However, the effects of enhanced demand for
cysteine on sulphate assimilation have not been addressed
in previous reports. Therefore, the enzyme activities and
steady-state mRNA levels of enzymes involved in sulphate
assimilation in poplars of wild type and those overexpressing the g-ECS in the cytosol (Noctor et al., 1996;
Arisi et al., 1997) were compared. As demonstrated in
Fig. 2, neither the activities nor the mRNA levels of
enzymes of the sulphate assimilation pathway were
affected in the leaves of the transgenic poplars. This result
shows that the capacity of sulphate reduction in poplars is
suf®cient to increase cysteine biosynthesis to the extent
necessary to accommodate enhanced GSH synthesis.
On the other hand, it was expected that the increased
GSH level might feedback±regulate ATPS and APR, as
described for several herbaceous plant species (Lappartient
et al., 1999; Vauclare et al., 2002). Inhibition of ATPS by
thiols is mediated by GSH in Brassica (Lappartient et al.,
1999); however, in maize, the mechanism is different and
the molecular signal is most probably cysteine (Bolchi
et al., 1999). Poplar, as a tree species with a long life span
and different needs for the regulation of nutrient supply
than the faster growing herbaceous plants, might easily
possess different regulatory elements (Herschbach, 2003)
and lack this kind of feedback regulation at all. It is,
therefore, signi®cant that exogenous feeding of GSH to the
roots caused the APR activity and mRNA accumulation to
decrease (Fig. 4). Thus, in poplar as well, GSH can exert
feedback regulation on APR and modulate the ¯ux through
the sulphate reduction pathway as described in the model
of demand-driven control of sulphate assimilation
(Lappartient and Touraine, 1996; Lappartient et al., 1999).
Why then were APR and ATPS not down-regulated by
even higher GSH concentration in the transgenic poplar
lines? The poplar lines used in the study are primary
transformants continuously propagated in vitro. One could
hypothesize that the plants adapted to the increased GSH
levels. Such adaptation would be possible only by
mutation in the promoter region of APR. However, the
probability that such mutation would occur in three
independent lines is extremely low. Also the subcellular
compartmentation of GSH most probably cannot explain
the contradictory results. In poplars overexpressing g-ECS
in the cytosol, the increase in total GSH could be ascribed
to the increase in the cytosolic GSH pool (Hartmann et al.,
2003); exogenous feeding with GSH would, primarily,
increase cytosolic GSH levels.
The simplest explanation for the contradictory data
would be the action of a second signal that positively
in¯uences APR mRNA accumulation and activity and
overrules the negative signal of GSH. A good candidate for
such a signal is OAS. OAS accumulates during sulphur
starvation and was thus proposed to act as a signal of the
sulphur status in seeds (Kim et al., 1999). APR activity was
induced in Lemna minor by feeding OAS both in the light
and in the dark (Neuenschwander et al., 1991). The
addition of OAS to nitrogen-deprived Arabidopsis led to
an increase in mRNA levels for APR, SIR, OASTL, and
SAT-A and to higher incorporation of 35So42± into thiols
and proteins (Koprivova et al., 2000). Increased synthesis
of OAS due to overexpression of SAT resulted in increased
cysteine and glutathione synthesis (Blaszczyk et al., 1999;
Harms et al., 2000). Most importantly, OAS de-repressed
sulphate transport and accumulation of mRNA for sulphate
transporters and, therefore, overrode a negative transcriptional control of the sulphate transporter (Smith et al.,
1997). Indeed, simultaneous feeding of GSH and OAS
abolished the feedback regulation of APR by GSH and
resulted in APR mRNA levels higher than in control plants
(Fig. 5). Accordingly, OAS levels were increased in leaves
of the transgenic line ggs5 and slightly, but not signi®cantly, elevated in ggs11 and ggs28 (Table 1). These
increased OAS contents might re¯ect sulphur depletion
caused by the increased GSH synthesis. However,
Herschbach et al. showed that, in the line ggs28, the
sulphate contents in leaves and roots and sulphate uptake
by the roots did not differ from the wild-type plants
(Herschbach et al., 2000) and, thus, there is no indication
of sulphur de®ciency causing the increase in OAS content.
On the other hand, OAS contents in the roots of transgenic
lines, did not follow an identical pattern. While in the ggs5
line the OAS level was lower than in wild-type plants, in
ggs11 and ggs28 plants the OAS concentration was not
different from the wild type; nevertheless, the APR activity
and mRNA level were not reduced by a highly elevated
GSH content (Fig. 3). Thus, although OAS contents
increased above physiological levels prevented feedback
regulation of APR by GSH, OAS cannot be the only
molecular signal in transgenic poplars responsible for
maintaining normal APR mRNA levels at elevated GSH
concentrations.
Apparently, poplars are able to distinguish between
GSH synthesized in the cell and GSH transported from
outside. In analogy to sugar-sensing by hexokinase, a
Regulation of sulphate assimilation in poplar 7 of 9
Acknowledgements
Fig. 5. Effect of OAS and GSH on APR activity and transcript level
in poplar roots. Poplar plants were treated by the addition of 1 mM
OAS, 1 mM GSH, or 1 mM OAS and 1 mM GSH to the normal
nutrition solution for 3 d. (A) Contents of cysteine (white bars) and
GSH (black bars) in roots were measured ¯uorometrically following
reduction by DTT, derivatization with monobromobimane, and
separation by reversed-phase HPLC. Mean values of 6SD of four
independent plants are presented. Different letters indicate values
signi®cantly different with P <0.05. (B) OAS contents were measured
by HPLC analysis. Mean values 6SD of three independent plants are
presented. Different letters indicate values signi®cantly different with
P <0.05. (C) APR activity was measured in roots extracts. Mean
values 6SD of four measurements are presented. (D) Total RNA (5
mg) isolated from roots of three plants, was separated on 1% agarose
in the presence of formaldehyde, blotted onto Hybond-N nylon
membrane, and hybridized with 32P-labelled APR cDNA fragments.
Relative APR mRNA levels were measured densitometrically and
normalized to rRNA. A value of 1.0 represents the mRNA level in
wild-type plants. Different letters indicate values signi®cantly different
with P <0.05.
signalling sucrose transporter, and hexose-transport-associated sensor (reviewed in Smeekens and Rook, 1997) two
hypotheses may be formed. Firstly, similar to hexokinase,
This work was ®nancially supported by the Deutsche
Forschungsgemeinschaft (DFG contract No. Re 515/11) as a part
of a research group FOR 383 `Sulphur metabolism in plants:
Junction of basic metabolic pathways and molecular mechanisms of
stress resistance'.
References
Arisi AC, Noctor G, Foyer CH, Jouanin L. 1997. Modi®cation of
thiol contents in poplars (Populus tremula3P. alba)
overexpressing enzymes involved in glutathione synthesis.
Planta 203, 362±372.
Blaszczyk A, Brodzik R, Sirko A. 1999. Increased resistance to
oxidative stress in transgenic tobacco plants overexpressing
bacterial serine acetyltransferase. The Plant Journal 20, 237±
243.
Bolchi A, Petrucco S, Tenca PL, Foroni C, Ottonello S. 1999.
Coordinate modulation of maize sulphate permease and ATP
sulphurylase mRNAs in response to variations in sulphur
nutritional status: stereospeci®c down-regulation by L-cysteine.
Plant Molecular Biology 39, 527±537.
Brunold C. 1990. Reduction of sulphate to sulphide. In:
Downloaded from http://jxb.oxfordjournals.org/ at Pennsylvania State University on February 20, 2013
in addition to catalytic activity g-ECS initiates a signalling
cascade that activates sulphate assimilation and/or prevents feedback inhibition by GSH. When GSH levels are
high enough to inhibit g-ECS (Hell and Bergmann, 1990),
the positive signal disappears and the feedback regulation
by GSH takes place. This hypothesis is unlikely since the
enzyme overexpressed in poplars is of bacterial origin and
its structure is completely different from its plant counterpart (May et al., 1998), so that such a mechanism would
have to be extremely well conserved evolutionarily. In the
second hypothesis, GSH transport across the plasma
membrane is a possible mechanism of the feedback
regulation of sulphate assimilation by GSH. The postulation of a signalling GSH transporter, similar to signalling
hexose and sucrose transporters in sugar sensing (reviewed
in Smeekens and Rook, 1997), would explain why APR
was down-regulated only by exogenously added GSH and
not in the transgenic poplars. Such a mechanism would
also be consistent with GSH transported from the leaves to
the roots via phloem acting as a signal of the sulphur status
(Lappartient and Touraine, 1996; Herschbach et al., 2000).
This hypothesis is compatible with all the data presented in
this report, taking into account the previous discussion of
OAS as a positive signal in APR regulation. However, this
hypothesis does not explain the inhibition of sulphate
uptake and assimilation by feeding cysteine, which must
be metabolized to GSH for this regulation (Lappartient and
Touraine, 1996; Vauclare et al., 2002). Clearly, these data
demonstrate that knowledge on the regulation of sulphate
assimilation is still partial. The exact nature of the
molecular mechanisms of the regulation of APR and
other enzymes of sulphate assimilation remains to be
elucidated.
8 of 9 Hartmann et al.
regulation of sulphur metabolism revealed through molecular and
genetic studies. Annual Review of Plant Physiology and Plant
Molecular Biology 51, 141±165.
Marzluf GA. 1996. Molecular genetics of sulphur assimilation in
®lamentous fungi and yeast. Annual Review of Microbiology 51,
73±96.
May MJ, Vernoux T, Leaver C, Van Montagu M, Inze D. 1998.
Glutathione homeostasis in plants: implications for environmental
sensing and plant development. Journal of Experimental Botany
49, 649±667.
Nakamura K, Hayama A, Masada M, Fukushima K, Tamura G.
1987. Measurement of serine acetyltransferase activity in crude
plant extracts by a coupled assay system using cysteine synthase.
Plant and Cell Physiology 28, 885±891.
Neuenschwander U, Suter M, Brunold C. 1991. Regulation of
sulphate assimilation by light and O-acetyl-L-serine in Lemna
minor L. Plant Physiology 97, 253±258.
Noctor G, Arisi A-CM, Jouanin L, Foyer CH. 1998b.
Manipulation of glutathione and amino acid biosynthesis in the
chloroplast. Plant Physiology 118, 471±482.
Noctor G, Arisi A-CM, Jouanin L, Kunert KJ, Rennenberg H,
Foyer CH. 1998a. Glutathione: biosynthesis, metabolism, and
relationship to stress tolerance explored in transformed plants.
Journal of Experimental Botany 49, 623±647.
Noctor G, Arisi A-CM, Jouanin L, Valadier M-H, Roux Y,
Foyer CH. 1997. The role of glycine in determining the rate of
glutathione synthesis in poplar. Possible implications for
glutathione production during stress. Physiologia Plantarum
100, 255±263.
Noctor G, Strohm M, Jouanin L, Kunert K-J, Foyer CH,
Rennenberg H. 1996. Synthesis of glutathione in leaves of
transgenic poplar overexpressing g-glutamylcysteine synthetase.
Plant Physiology 112, 1071±1078.
Rolletschek H, Hajirezaei MR, Wobus U, Weber H. 2002.
Antisense-inhibition of ADP-glucose pyrophosphorylase in Vicia
narbonensis seeds increases soluble sugars and leads to higher
water and nitrogen uptake. Planta 214, 954±964.
Schmutz D, Brunold C. 1982. Rapid and simple measurement of
ATP-sulphurylase activity in crude plant extracts using an ATP
meter
for
bioluminescence
determination.
Analytical
Biochemistry 121, 151±155.
Schneider S, Bergmann L. 1995. Regulation of glutathione
synthesis in suspension cultures of parsley and tobacco.
Botanical Acta 108, 34±40.
Schupp R, Rennenberg H. 1988. Diurnal changes in glutathione
content of spruce needles (Picea abies L.). Plant Science 57, 113±
117.
Smeekens S, Rook F. 1997. Sugar sensing and sugar-mediated
signal transduction in plants. Plant Physiology 115, 7±13.
Smith FW, Hawkesford MJ, Ealing PM, Clarkson DT, van
den Berg PJ, Belcher AR, Warrilow AG. 1997. Regulation
of expression of a cDNA from barley roots encoding a high
af®nity sulphate transporter. The Plant Journal 12,
875±884.
Strohm M, Jouanin L, Kunert KJ, Pruvost C, Polle A, Foyer
CH, Rennenberg H. 1995. Regulation of glutathione synthesis in
leaves of transgenic poplar (Populus tremula3P. alba)
overexpressing glutathione synthetase. The Plant Journal 7,
141±145.
Suter M, von Ballmoos P, Kopriva S, Op den Camp R, Schaller
J, Kuhlemeier C, SchuÈrmann P, Brunold C. 2000. Adenosine
5¢-phosphosulphate sulphotransferase and adenosine 5¢phosphosulphate reductase are identical enzymes. Journal of
Biological Chemistry 275, 930±936.
Vauclare P, Kopriva S, Fell D, Suter M, Sticher L, von Ballmoos
P, KraÈhenbuÈhl U, Op den Camp R, Brunold C. 2002. Flux
Downloaded from http://jxb.oxfordjournals.org/ at Pennsylvania State University on February 20, 2013
Rennenberg H, Brunold C, De Kok LJ, Stulen I, eds. Sulphur
nutrition and sulphur assimilation in higher plants. The Hague,
The Netherlands: SPB Academic Publishing, 13±31.
Brunold C, Suter M. 1990. Adenosine 5¢-phosphosulphate
sulphotransferase. In: Lea P, ed. Methods in plant biochemistry,
Vol. 3. London: Academic Press, 339±343.
Foyer CH, Rennenberg H. 2000. Regulation of glutathione
synthesis and its role in abiotic and biotic stress defence. In:
Brunold C, Rennenberg H, De Kok LJ, Stulen I, Davidian JC, eds.
Sulphur nutrition and sulphur assimilation in higher plants:
molecular, biochemical and physiological aspects. Bern,
Switzerland: Paul Haupt, 127±153.
Grif®th OW. 1982. Mechanism of action, metabolism, and toxicity
of buthionine sulphoximine and its higher homologs, potent
inhibitors of glutathione synthesis. Journal of Biological
Chemistry 257, 13704±13712.
Harms K, von Ballmoos P, Brunold C, Hofgen R, Hesse H. 2000.
Expression of a bacterial serine acetyltransferase in transgenic
potato plants leads to increased levels of cysteine and glutathione.
The Plant Journal 22, 335±343.
Hartmann T, Mult S, Suter M, Rennenberg H, Herschbach C.
2000. Leaf age-dependent differences in sulphur assimilation and
allocation in poplar (Populus tremula3P. alba) leaves. Journal of
Experimental Botany 51, 1077±1088.
Hartmann TN, Fricker MD, Rennenberg H, Meyer AJ. 2003.
Cell-speci®c measurement of cytosolic glutathione in poplar
leaves. Plant, Cell and Environment 26, 965±975.
Hell R, Bergmann L. 1990. g-Glutamylcysteine synthetase in
higher plants: catalytic properties and subcellular localization.
Planta 180, 603±612.
Herschbach C. 2003. Whole plant regulation of sulphur nutrition of
deciduous trees ± In¯uences of the environment. Plant Biology 5,
215±340.
Herschbach C, van Der Zalm E, Schneider A, Jouanin L, De
Kok LJ, Rennenberg H. 2000. Regulation of sulphur nutrition in
wild-type and transgenic poplar over-expressing gammaglutamylcysteine synthetase in the cytosol as affected by
atmospheric H2S. Plant Physiology 124, 461±473.
Jones-Mortimer MC. 1973. Mapping of structural genes for the
enzymes of cysteine biosynthesis in Escherichia coli K12 and
Salmonella typhimurium LT2. Heredity 31, 213±221.
Kim H, Hirai MY, Hayashi H, Chino M, Naito S, Fujiwara T.
1999. Role of O-acetyl-L-serine in the coordinated regulation of
the expression of a soybean seed storage-protein gene by sulphur
and nitrogen nutrition. Planta 209, 282±289.
Kopriva S, BuÈchert T, Fritz G, et al. 2002. The presence of an
iron-sulphur cluster in adenosine 5¢-phosphosulphate reductase
separates organisms utilizing adenosine 5¢-phosphosulphate and
phosphoadenosine 5¢-phosphosulphate for sulphate assimilation.
Journal of Biological Chemistry 277, 21786±21791.
Koprivova A, Suter M, Op den Camp R, Brunold C, Kopriva S.
2000. Regulation of sulphate assimilation by nitrogen in
Arabidopsis. Plant Physiology 122, 737±746.
Lappartient AG, Touraine B. 1996. Demand-driven control of
root ATP sulphurylase activity and So42± uptake in intact canola.
The role of phloem-translocated glutathione. Plant Physiology
111, 147±157.
Lappartient AG, Vidmar JJ, Leustek T, Glass ADM, Touraine
B. 1999. Inter-organ signalling in plants: regulation of ATP
sulphurylase and sulphate transporter genes expression in roots
mediated by phloem-translocated compound. The Plant Journal
18, 89±95.
Lee S, Leustek T. 1999. The affect of cadmium on sulphate
assimilation enzymes in Brassica juncea. Plant Science 141, 201±
207.
Leustek T, Martin MN, Bick JA, Davies JP. 2000. Pathways and
Regulation of sulphate assimilation in poplar 9 of 9
control of sulphate assimilation in Arabidopsis thaliana:
adenosine 5¢-phosphosulphate reductase is more susceptible to
negative control by thiols than ATP sulphurylase. The Plant
Journal 31, 729±740.
von Arb C, Brunold C. 1983. Measurement of ferredoxindependent sulphite reductase activity in crude extracts from
leaves using O-acetyl-L-serine sulphhydrylase in a coupled assay
to measure the sulphide formed. Analytical Biochemistry 131,
198±204.
Westerman S, Stulen I, Suter M, Brunold C, De Kok JL. 2001.
Atmospheric H2S as sulphur source for Brassica oleracea:
consequences for the activity of the enzymes of the
assimilatory sulphate reduction pathway. Plant Physiology and
Biochemistry 39, 425±432.
Downloaded from http://jxb.oxfordjournals.org/ at Pennsylvania State University on February 20, 2013