Artificial Sun based on Light
advertisement

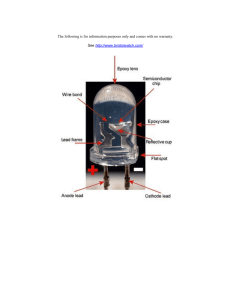
Artificial Sun based on Light-emitting Diode Siyu Chen, 22/05/2015 493989910@qq.com Department of Physics, Xiamen University 1. INTRODUCTION Electroluminescence as an optical phenomenon was discovered in 1907 by the British experimenter H. J. Round of Marconi Labs. After that many scientists devoted to important photonic technologies that are changing the world around us by enabling new applications or transforming existing ones. The “Light Emitting Diode” or LED as it is more commonly called, seems to be a typical and important product since then. Over the past forty years, advances in materials science have enabled these compound semiconductors rapidly becoming a brand new light source which emits a bright, efficient light that is giving cars, interior lighting and signage a radical new look. However, although LEDs break the restriction of traditional luminescent mechanism and has many advantages such as the high stability, the wide controllable output range as well as the long life, there exist some defects. One of serious defects is that compared with traditional halogen and xenon lamps which can cover a wide spectral bandwidth, the spectrum of LED is usually monochromatic, which is quite different from solar spectrum. In this article, I present an original approach to combine numerous multiple color LEDs to fabricate an artificial sun. It functioned well as a mini sun with the features of low cost and easy implementation. I believe that this light source that reproduces the solar spectrum and power will be vital for animal, plant and solar devices in the future. 2. THEORY AND FOUNDATION 2.1. Semiconductor material As a matter of fact, the popular LED is basically just a specialized type of diode made from a very thin layer of fairly heavily doped semiconductor material. In a semiconductor material, such as silicon, the atoms have 4 electrons in the outermost electron energy band, called the valence band. This leaves room for 4 more electrons in the valence band, and these available "electron-slots", often referred to as holes. By introducing atoms with different electron-configurations into the lattice we can create a doped semiconductor. If we introduce trace amounts of the boron atom to a silicon lattice we get a p-type semiconductor. Because boron only has three valence electrons, this will leave occasional holes in the valence bands in the lattice. At room temperature these holes will move quite freely around in the material effectively making it a positive charge carrier, hence the name "p-type" semiconductor. Similarly an n-type semiconductor can be formed by doping the same silicon lattice with arsenic. Arsenic has 5 valence electrons and will provide the material with easily movable electrons at room temperature, which is a negative charge carrier, hence the name "n-type" semiconductor. Fig.1 1. (a) P-ttype semicon nductor (b) N-type semico onductor 2.2. LED Inner workinggs When a piec ce of semiconductor matterial change es type from m p-type to n-type over a cross-sectio on it forms a P-N junction n. At tempera atures well above a absolu ute zero, hole es from the p-side p of the P-N junction n wander into o the n-side d due to thermal vibrations s. At the sam me time electrons from the n-side wan nder into the e p-side. This s leaves an arrea over the junction pre etty much fre ee of charge--carries calle ed the deplettion area. Wh hen given an n external app plied voltage e, continuous electrons e encounter ho oles in the depletion are ea. They will fill the holes s and release energy in th he form of a photon. Fig.2. Inn ner workings s of an LED, showing circ cuit (top) and band diag ram (bottom m) 3. EXPERIM MENTS (a a mere fiction) 3.1. Spectru um of sunligh ht As a first sttep, in order to obtain the main fre equency ingredient of the uneven ssolar irradiation, Fourierr transform m method was used to analyze solar sp pectrum and d to calculate e the intens ity distributiion. Because e the Earth's atmosphere blocks out a broad range of the sun spectrum, only allowing a narrow band of light to reach the surface, most of the energy of radiation that sun emits is in the visible, near-infrared and near-ultraviolet region. Therefore, I focus on the wavelength range from ultraviolet light (330 nm) through visible light to infrared light (930 nm) and extract the 6 main component of the frequencies in sunlight spectrum—They are infrared(710 nm), red(650 nm), yellow(580 nm), green(530 nm), blue(470 nm) and ultraviolet(390 nm) respectively. 3.2. Colors and I-V characteristic As the above theories in section 2 proved, the wavelength of photons emitted, and also the color of light, depends on the band gap energy and structure of the materials forming the P-N junction. According to Table 1 from Wikipedia, I chose and compounded 6 kinds of materials which have a direct band gap with energies corresponding to near-infrared, visible, and near-ultraviolet light used for the LED in the laboratory for Physics at Xiamen University. No matter what colors of LEDs are, they are operated from a low voltage DC supply with a series resistor used to limit the forward current to prevent destruction by overheating in my experiment. These LEDs used for artificial sun emit light when approximately 5mA current flow through it and can withstand 30mA or more current where a high brightness light output is needed. Each LED was applied its own forward voltage across the PN junction for a specified amount of forward conduction current (typically 20mA) and this parameter which is determined by the semiconductor material. Color Wavelength [nm] Infrared λ > 760 Semiconductor material Gallium arsenide (GaAs) Aluminium gallium arsenide (AlGaAs) Aluminium gallium arsenide (AlGaAs) Red 610 < λ < 760 Gallium arsenide phosphide (GaAsP) Aluminium gallium indium phosphide (AlGaInP) Gallium(III) phosphide (GaP) Gallium arsenide phosphide (GaAsP) Orange 590 < λ < 610 Aluminium gallium indium phosphide (AlGaInP) Gallium(III) phosphide (GaP) Gallium arsenide phosphide (GaAsP) Yellow 570 < λ < 590 Aluminium gallium indium phosphide (AlGaInP) Gallium(III) phosphide (GaP) Gallium(III) phosphide (GaP) Green 500 < λ < 570 Aluminium gallium indium phosphide (AlGaInP) Aluminium gallium phosphide (AlGaP) Zinc selenide (ZnSe) Blue 450 < λ < 500 Indium gallium nitride (InGaN) Silicon carbide (SiC) Violet 400 < λ < 450 Indium gallium nitride (InGaN) Diamond (C) Ultraviolet λ < 400 Aluminium nitride (AlN) Aluminium gallium nitride (AlGaN) Aluminium gallium indium nitride (AlGaInN) Table 1 Available colors with wavelength range, and semiconductor material 3.3. Structure of LED Through advanced technology in microelectronics, I successfully make a large number of LED devices with ever-shorter wavelengths, emitting light in a variety of colors. The detailed construction of my Light Emitting Diode is very different from that of a normal diode. The PN junction of it is surrounded by a transparent, hard plastic epoxy resin hemispherical shaped shell or body which protects the LED from both vibration and shock. LED chip as the core consists of a chip of semiconducting material doped with impurities. It is fixed on the column and powered by current from the positive lead frame to negative one. Although not directly labeled, the anvil and post act as anchors, to prevent the conductors from being forcefully pulled out from mechanical strain. 3.4. LED lattice and IRGBU system As we know, white light can be formed by mixing red, green and blue lights. Therefore, it is my belief that the invention of the blue LED made possible a simple and effective way to generate sunlight, combining red, yellow, green, blue, near-infrared, and near-ultraviolet LEDs. My arterial sun is born by utilizing this principle. It is constituted by IRGBU system I invented which is an extension of the RGB system. Each of IRGBU system consists of one near-infrared, one red, one yellow, one green, one blue, and one near-ultraviolet LED. These six LEDs with different wavelengths are capable of producing any color and this mixture of colored light will be perceived by humans as sunlight and can be used for general illumination even providing energy for plants to photovoltaics. But several key factors in my method including color stability, color rendering capability, and luminous efficacy need a strict adjustment because higher efficiency often means lower color rendering. After repeated attempts I finally presented a trade-off between the luminous efficiency and color rendering. Fig.3 3. (a) illuminant schem matic (b) Structure of LED la attice Inspired by structure of graphene, I set every 6 LEDs of diffe erent wavele engths on the e regular hex xagon lattice e constructed in a honeycomb. Initially, I arrange all LED on th he same plan ne like displa ay to ensure that incidentt light require es illuminatin ng the whole e object as fla at as possiblle. The schem matic illustra ation of the equipment e is s shown in Fig g. 3. The equ uipment has 6 different w wavelength chip-type LED Ds, that colorrs are red, ye ellow, green,, blue, infrare ed, ultraviolet. I assume ed that thesse LEDs are put in the hexagon h as a unit, and the distance e between the e same colorrs is 12 mm and that be etween each LED is 4 mm m. The exam mple of LED arrangement a t is shown in Fig.3.(b). The theorettical value off luminous in ntensity is ass follows: Where I(x,y y) is the irrad diance at the e measurem ent point, n is a numberr of LEDs, Ils is the irradiance of LED,, θ and r are the angle an nd the distan nce from the e light source e to the mea asurement p point, shown in Fig.3.(a). The last step is to warp the plane LED lattice in nto a global as a if process s graphene in nto a Bucky Ball. At the same time, a high-perfo ormance batttery and a ccontrol syste em of circuit is hidden in this global to t drive the whole artific cial sun. Fig.4 4. The progre ess of fabric cated an artifficial sun 4. RESU ULT AND DISCUSSIION (a merre fiction) Finally, I tes sted this optical system of o multi-LED Ds so that ens sure its spec ctral power d distribution simulates s the e sunlight acc curately. Using spectrogrraph, the em mission specttrum line of my artificiall sun is measured. From m the figure below we can n see that the curve of L LED spectrum m is close to the curve off real sunligh ht spectrum. As a result, artificial solar irradiation is confirme ed. Fig.5. Sun spectrum vs LED spectrum 5. CONCLUSION As everyone knows, solar energy as an essential power source is providing energy for everything from plants to photovoltaics. My method employs different LED arrays driven by constant current source and successfully makes the output irradiance reliable and closes to the sun. By adjusting the parameter of control system, we can achieve different distribution forms of output spectrum to meet the demands of spectral matching degree in different areas. Using cage structure light source of LEDs instead of traditional lamp will also greatly simplify the optical system and lower the manufacturing cost. In short, I successfully fabricated an artificial sun based on LEDs and the capability of this equipment was examined in the experiment, which means that I can illuminate an object like an actual sun. This simple system for emitting high-quality solar light will be useful for next-generation lighting systems. I believe that my artificial sun will play extensively an important role in solar thermal systems, photo catalysts, and plant factories. ACKNOWLEDGEMENT I express appreciation for fruitful discussions with my roommates Xi-hao Chen and Yi-nong Chen, and thanks for my specialized English teacher Tien-Mo Shih giving me a great deal of help and Wikipedia offering relevant data. REFERENCE • Haitz's law". doi:10.1038/nphoton.2006.78. Retrieved 2009-03-04. • en.wikiversity.org/wiki/PN_Junction • "The life and times of the LED: a 100-year history". Nature Photonics 1 (4): 189–192. doi:10.1038/nphoton.2007.34