Slides
advertisement

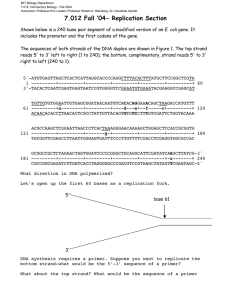
When does replication occur? • Full blown replication only occurs MBLG1001 lecture 10 Replication… the once in a lifetime event! The once in a lifetime process • • • • This is a very tightly regulated process It must be coordinated with cell division The whole genome is faithfully copied. The process must be as error free as possible as this is the template for the next generation. The replication forks once, just before cell division • BUT the DNA template is constantly being repaired. • There are some 130 separate genes for DNA repair in your genome which work away in the background all the time. The problems revisited: • Replication is bi-directional. The theta model. • Bacterial DNA is a closed circle so it will get tangled when it is unwound. • DNA polymerases only work in one direction and need primers • The strands must be pulled apart and unwound. Summarising what we know: oriC • Initiation is tightly regulated at one site. • 2 replication forks start in opposite directions from this site • Elongation is semi-conservative, proved by the Messelson Stahl experiment, carried out by DNA polymerases 1 Initiation • E coli has one defined site, oriC, origin of replication on the chromosome or replicator • There is a sequence, 200 – 300 bp long which is recognised by the initiator protein DnaA • 20 – 30 DnaA monomers bind to oriC in a coordinated ATP-dependent (needs energy) fashion and recruit DnaB, a helicase, Summarising In prokaryotes (bacteria) • One site • A specific sequence • Specific proteins bind in a specific order • Eukaryotes have multiple chromosomes and multiple oriC sites. Helicase or DnaB Helicase or DnaB • DnaB or helicase is a hexameric protein (6 subunits) which unwinds double stranded DNA. This is actually a whole class of enzymes (12 types occur in E. coli alone) present in all cells. • It first arrives in the initiation complex and is present throughout replication • To replicate, repair or transcribe DNA you need access to the middle of the double helix, where the information is stored. • Helicases break the h-bonds (not the covalent phoshpodiester sugar phosphate backbone) thus separating the two strands. It requires energy to perform this function, hydrolyzing NTPs to NDPs + Pi. Once the strands are apart.. We need to stop them tangling.. • They need to be kept apart • This is done by single stranded binding protein (ssbp) • Single stranded binding protein (ssbp) prevents the two strands reannealing, as well as forming intrastrand loops and nuclease attack. The E. coli ssbp is a tetramer which binds to the single stranded DNA melted by helicase. • This is particularly a problem with a closed circle. As you unravel the 2 strands at one end they will become increasingly tangled at the other end. • This is solved with a class of proteins known as topoisomerses. 2 The tangling problem • Topoisomerases. These enzymes ‘untangle’ the DNA in the regions still to be copied. • There are 2 types of topoisomerases; type I and type II. • Type I topoisomerases cut one strand of the backbone, allow the other strand to pass through the gap then reform. Negative and positive supercoiling The tangling problem • Type II Topoisomerases. DNA gyrase is the most famous. Clark p107 Drugs used • It cuts both strands and introduces negative supercoiling to the DNA. • It requires an energy source. (Type I enzymes get their energy from the potential energy stored in the stressed DNA) • DNA is normally in a state of slight negative supercoiling. Why? We need a primer to get going.. Clark p89 Malacinski p39 • Negative supercoiling is when the 2 strands of DNA have twisting energy applied in the opposite direction to the twist of the helix. The double helix is then easier to melt. • Positive supercoiling is when the 2 strands have twisting energy applied in the same direction as the helix. The strands are more difficult to pull apart. • Primase is an RNA polymerase and is part of the primosome complex. It produces short RNA primers (11-12 nt) for DNA pol III at initiation and in the lagging strand. The elephant in the room The elephant in the room! • How do we get around the fact that the replication fork is copying both strands simultaneously but the enzyme only works in one direction! • Enter the leading and lagging strands. • One strand is easy…this is the leading strand. It is the parent strand in the orientation 3’ to 5’ 3 Replication Fork Replication Fork Direction of fork movement DNA gyrase Helicase Primase Replication Fork Replication Fork The 2 assemblies of DNA pol III are tethered together Single stranded binding protein DNA polymerase III subassembly The leading strand The leading strand Parent strand 5’P 3’OH dNTP HO Parent strand 5’P 5’P 3’OH 3’OH • DNA polymerase simply works its way around the chromosome on this strand 5’P Newly synthesised strand 4 Newly synthesised primer Replication Fork The lagging strand 5’P Newly synthesised DNA • The template must wrap around and approach the DNA pol III assembly from the opposite direction • It is synthesised discontinuously in short stretches known as Okazaki fragments, each with a new primer • The primers are removed and the fragments join up RNA primer 3’OH 5’P 5’P Previously synthesised primers Replication Fork The journey analogy 5’P Newly synthesised DNA 5’P RNA primer Lagging strand synthesis 3’OH 5’P dNTPs 3’OH Leading strand • If replication is taking the equivalent of a 400 k journey in 40 min with an error every 170 k imagine this journey is taken by 2 delivery trucks • One truck drives the route non-stop. • The other truck stops every second and a delivery person must take a detour off the road before rejoining it again! 5’P Processing the lagging strand Sealing the nick…. • Once the Okazaki fragment has been made DNA pol I comes in, removes the primer (with its 5’ to 3’ exonuclease activity) and fills in the gap (with its 5’ to 3’ polymerase activity). • The nick is sealed by DNA ligase. • DNA ligase seals nicks in the sugar phosphate backbone, reforming the phosphodiester bond. • To do this it needs a source of energy. In E. coli this is provided by NAD; in higher organisms ATP is the energy source. DNA pol I can’t join the nick as it relies on the hydrolysis of pyrophosphate from dNTPs to form the phosphodiester bond. 5 Gap sealed with DNA ligase DNA pol I Using its 5’ to 3’polymerase and its proof reading 3’ exonuclease activity 5’P 3’ nucleotides OH 5’ Using its 5’ to 3’ exonuclease activity it will chew up the RNA primer before it. This is also known as its intrinsic RNaseH activity Figure 28.10 General features of a replication fork. The DNA duplex is unwound by the action of DNA gyrase and helicase, and the single strands are coated with SSB (ssDNA-binding protein). Primase periodically primes synthesis on the lagging strand. Each half of the dimeric replicative polymerase is a “core” polymerase bound to its template strand by a β-subunit sliding clamp. DNA polymerase I and DNA ligase act downstream on the lagging strand to remove RNA primers, replace them with DNA, and ligate the Okazaki fragments. Figure 28.6 The semidiscontinuous model for DNA replication. Newly synthesized DNA is shown as red. Because DNA polymerases only polymerize nucleotides 5 3, both strands must be synthesized in the 5 3 direction. Thus, the copy of the parental 3 5 strand is synthesized continuously; this newly made strand is designated the leading strand. (a) As the helix unwinds, the other parental strand ( the 5 3, strand) is copied in a discontinuous fashion through synthesis of a series of fragments 1000 to 2000 nucleotides in length, called the Okazaki fragments; the strand constructed from the Okazaki fragments is called the lagging strands. (b) Because both strands are synthesized in concert by a dimeric DNA polymerase situated at the replication fork, the 5 3 parental strand must warp around in trombone fashion so that the unit of the dimeric DNA polymerase replicating it can move along it in the 3 5 direction. This parental strand is copied in a discontinuous fashion because the DNA polymerase must occasionally dissociate from this strand and rejoin it further along. The Okazaki fragments are then covalently joined by DNA ligase to form an uninterrupted DNA strand. 6