Clinigen Group Full Year Results FY15 and
Proposed Acquisition of Link Healthcare
September 2015
Peter George (CEO), Shaun Chilton (Deputy CEO),
Robin Sibson & Martin Abell (CFO)
Group overview
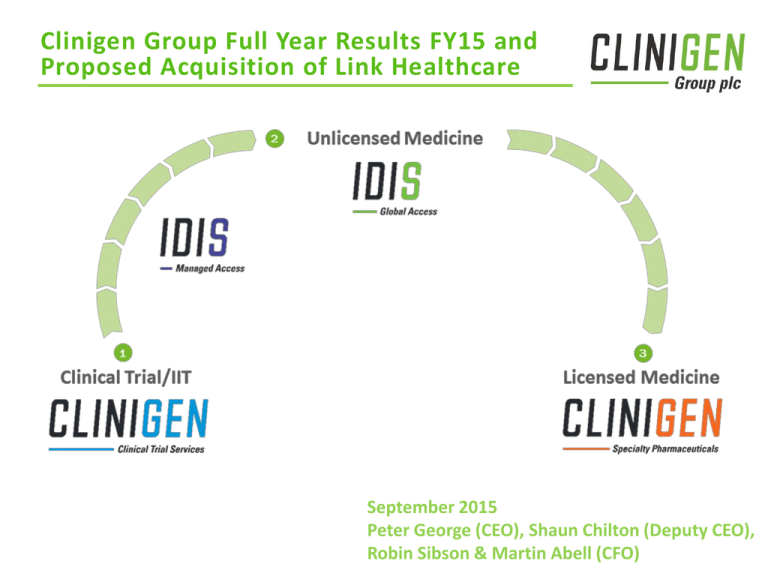
Global specialty pharmaceuticals and services business, supplying clinical trial, licensed and unlicensed
critical, life saving drugs
Four distinct operating businesses which benefit from important synergies between them:
Clinigen CTS – Global leader in the specialist supply and management of quality-assured medicines for
patients in clinical trials
Idis MA – Global leader in ethical worldwide access to the most promising innovative early stage
medicines on behalf of pharma and biotech companies to meet an unmet patient need
Idis GA – Ethical Supply of unlicensed or short supply medicines to patients via their physicians
Clinigen SP – SP acquires the rights to and then revitalises essential niche hospital only medicines and
has a portfolio of oncology support and infectious disease medicines
Global network serving 130 countries with a broad, blue chip customer base of global
pharmaceutical/biotech companies and contract research organisations
Highly profitable, cash-generative business with impressive continuous growth over the past five years
Good visibility of earnings with significant growth opportunities, both organically and through acquisition
© Copyright Clinigen Group plc. All rights reserved
Right Drug Right Patient Right Time
2
FY15 results - key highlights
Financial Highlights
Revenues increased in excess of 45% to £184.4m (FY14: £126.6m), +27% on proforma basis
Gross profit increased 30%, mainly driven by greater than 25% growth in Specialty
Pharmaceuticals (SP) gross profits and the acquisition of Idis
Underlying EBITDA* up 20% to £32.3m (FY14: £26.8m)
Final dividend of 2.3 pence per share proposed, bring the total dividend to 3.4 pence per share
(FY14 3.1 pence per share)
Business Highlights
Clinigen acquired Idis April 2015; creating the global market leader in both the ethical supply of
unlicensed medicines and access to short supply medicines.
Proposed acquisition of Link Healthcare strengthens Clinigen’s global footprint across Asia,
Africa and Australasia, allowing access to more healthcare professionals around the world to the
medicines they need for their patients
The Group’s owned oncology support portfolio was strengthened with the acquisition of Ethyol®
(amifostine) from AstraZeneca in August 2014
Underlying
EBITDAGroup
& EPSplc.
adjusted
to reserved
exclude amortisation
©*Copyright
Clinigen
All rights
Right Drug Right Patient Right Time
3
FY15 results - key highlights
Clinigen Clinical Trial Services (CTS)
CTS developing new Expanded Value Services; “Just in Time” smarter supply and labelling
and direct to site services
Idis Managed Access (MA)
Increase in deliveries to 418,000 units, through 62,000 shipments; up from 263,000 units
across 40,000 shipments in FY14
As the market leader; in FY15 we have 99 products under active management with 19 of
the top 25 pharma and biotech companies, shipping to 95 countries
Idis Global Access (GA)
The acquisition of Idis has created this new business unit
Idis GA together with Link Healthcare creates huge potential to dominate and shape the
ethical on-demand unlicensed supply market, estimated at $5-7bn.
Clinigen Specialty Pharmaceuticals (SP)
Foscavir “risk” diluted by new products, now 70% of SP sales and profit, significantly
reduced from 86% at the end of FY14.
Completed the transfer of market authorisations for Cardioxane from Novartis, Savene from
Norgine and Ethyol from Astra Zeneca.
Strategic Alliance with Cumberland Pharmaceuticals will provide support for Clinigen
products in the US, with Clinigen supporting Cumberland outside the US.
© Copyright Clinigen Group plc. All rights reserved
Right Drug Right Patient Right Time
4
Income statement 2015
£m
year ended 30 June
Sales
Gross profit
Gross profit margin (%)
Overheads1
2015 Gross profit (£53.7m)
2014
2015 Growth
126.6
184.4
46%
41.2
33%
-14.4
53.7
29%
-21.4
EBITDA1 (underlying)
Margin (%)
26.8
21%
32.3
18%
20%
Pre tax profit (underlying)1
23.1
26.2
13%
24.5p
28.0p
14%
3.1p
3.4p
10%
Earnings per share (underlying)2
Total dividend
30%
Audited
GP
49%
1: Overheads, EBITDA & underlying PBT represent underlying performance, see slide 8 for reconciliation to reported
© Copyright
plc. on
All underlying
rights reserved
2: Adjusted
earnings Clinigen
per shareGroup
is based
PAT adjusted to exclude amortisation and associated tax
Proforma 2015
Gross profit
Proforma
GP
Right Drug Right Patient Right Time
5
Group balance sheet
Goodwill and intangibles increase driven by Idis
acquisition (£240m) and Ethyol, also
incorporates full writedown of Vibativ £3.4m
Current working capital requirement remains
low
£140m debt facility in place (term loan £45m
and RCF £95m) until April 2020, utilisation at
June 2015; £106m
Net debt of £78m will increase on completion of
Link acquisition and expected to reduce to
current levels in FY16
Opening fair value adjustments amounted to
£91.8m, the main element being the valuations
assigned to brand/contracts/clients relationships
£m
Year ended 30 June
Property, plant and equipment
Goodwill & other intangible assets
Deferred tax asset
Total non-current assets
2014
2015
1.0
50.5
1.9
53.4
1.6
308.2
3.9
313.7
2.5
27.1
21.8
51.4
11.1
67.1
27.8
106.0
Total assets
104.8
419.7
Trade and other payables
Loans and borrowings
Current and deferred tax
Provisions
Total current liabilities
(19.5)
(87.7)
(16.5)
(69.5)
(2.5)
(2.9)
(1.5)
(38.5) (161.6)
Inventories
Trade and other receivables
Cash and cash equivalents
Total current assets
Deferred tax
Loans and borrowings
Total non-current liabilities
Total liabilities
Total equity
© Copyright Clinigen Group plc. All rights reserved
-
(19.0)
(34.5)
(53.5)
(38.5) (215.1)
66.3
204.6
Right Drug Right Patient Right Time
6
Group cash flow
Strong cash generation from underlying
operations and £12.1m after tax and
non underlying items
Purchase of Intangibles of £188.1m is
£180m Idis and £8m Ethyol
£106m drawn down on new facility in
April 2015 to fund Idis acquisition. Loan
advances reflect this offset by
settlement of previous Clinigen and Idis
loans of £16.5m and £36m
27,000,000 new ordinary shares issued
April 2015 at a price of 500 pence per
share to fund Idis acquisition
© Copyright Clinigen Group plc. All rights reserved
£m
2014
2015
16.2
5.3
Add: Corporation tax
5.1
3.1
Interest paid
0.2
0.8
D&A, impairment and loss on disposal
3.5
12.2
Share based payment expense
1.2
1.3
Year ended 30 June 2013
Profit after tax
(Increase)/decrease in net working capital
Increase in provisions
(5.5) (10.2)
-
1.5
Income taxes paid
(1.1)
(1.9)
Net cash flows from operating activities
19.6
12.1
Purchase of PP&E
(0.6)
(0.2)
Purchase of intangibles
(21.8) (188.1)
Net cash used in investing activities
(22.4) (188.3)
Interest paid
(0.2)
(0.8)
Loan (repayments) and advances
16.5
53.3
Dividends
(2.5)
(2.6)
Issue/(purchase) of shares
Net cash generated from financing
activities
(0.3) 132.4
Total cash flows
10.7
13.5 182.3
6.1
Right Drug Right Patient Right Time
7
Reconciliation for EBITDA to PAT
Underlying
Reported
£m’s
Underlying EBITDA
Interest expense
D&A, impairment and disp. loss
Underlying PBT
2014
26.8
(-0.2)
(-3.5)
23.1
2015
32.3
(-0.8)
(-5.3)
26.2
2014
26.8
(-0.2)
(-3.5)
23.1
2015
32.3
(-0.8)
(-5.3)
26.2
Share based payment charge
Impairment/amortisation of intangibles
Idis acquisition costs/restructuring
PBT
Taxation (underlying)
Taxation (non-underlying)
PAT
23.1
(-5.4)
26.2
(-5.7)
17.6
20.4
(-1.8)
21.3
(-5.4)
0.4
16.2
(-2.3)
(-5.9)
(-9.5)
8.4
(-5.7)
2.6
5.3
© Copyright Clinigen Group plc. All rights reserved
Right Drug Right Patient Right Time
8
Operating Businesses
Clinigen Clinical
Trial Services
Clinigen has consolidated its global market leader
position with the acquisition of Idis
CTS remains the main revenue generator in the Group
with 61% of sales (38% proforma), with the lowest GM%
Addressable outsourced drug sourcing market c. $2.5bn,
estimated to grow at 8% over next 3 years, with
expanded value services market a further c.$1bn
Introduction of expanded value services; “Just in Time” smarter supply and labelling and “direct
to site” services, will improve margins during FY16
30-50% of purchased clinical trial drugs are wasted, due to poor purchasing decisions; “Just in time”
services aimed at reducing this waste
Increasing demand for Real World Data driving demand for labelling and direct to site services
Currently running direct to site projects for 8 companies, on 22 clinical studies, supplying 33
medicines to 252 sites
© Copyright Clinigen Group plc. All rights reserved
Right
DrugRight
RightPatient
PatientRight
RightTime
Time
Right
Drug
10
Clinigen Clinical
Trial Services
£m
Clinigen CTS
Audited
12 months ended 30 June FY15
Sales
112.7
YoY Growth %
34%
Gross Profit
13.4
YoY Growth %
7%
Gross Profit Margin (%)
12%
Audited Pro forma Pro forma Pro forma
FY14
FY15
FY14
FY13
83.6
134.8
93.0
92.9
12.6
15%
45%
0%
16.2
13.6
19%
13%
12%
15%
12.0
13%
Financial Year 2015
Sales up 34% and an even better 45% on a proforma basis
Gross profit performance, up 7% (19% proforma) at a 12% gross
margin. This will improve with expanded value services
Underlying activity up, with medicines supplied up 13% and number
customers up from 73 to 85
Sales pipeline strong @ c.£137m, H1 pipeline of 80-100% probability
is at £42.8m, GM 14.7% (Prior Year £20.6m)
Deeper customer presentation
7 customers with sales greater than £5m, up from 5 in FY14
Half of the top 25 pharmaceutical companies, by R&D spend,
are CTS customers
© Copyright Clinigen Group plc. All rights reserved
Right
DrugRight
RightPatient
PatientRight
RightTime
Time
Right
Drug
11
Idis Managed Access
Idis MA is the global market leader in the exclusive managed access to the most promising
innovative early stage medicines on behalf of pharma and biotech companies
The Idis acquisition allowed the Group to combine the Idis MAP and Clinigen GAP businesses
under the Idis Managed Access (MA) brand.
The integration has fully amalgamated these businesses with immediate effect and
seamlessly, post-acquisition
Proforma FY15 has seen an increase to 418,000
units delivered through 62,000 shipments; up
from 263,000 units across 40,000 shipments in
FY14
Idis MA is the market leader in this rapidly
evolving sector valued at c.$500-600m
Idis MA has 99 products under active
management, is working with 19 of the top
25 pharma and biotech companies, and over
50 in total, and distributed unlicensed
medicines to 95 countries in FY15.
© Copyright Clinigen Group plc. All rights reserved
Right Drug Right Patient Right Time
15
12
Idis Managed Access
Idis MA
£m
12 months ended 30 June
Sales
Audited
FY15
28.8
YoY Growth %
78%
Gross Profit
8.3
YoY Growth %
53%
Gross Profit Margin (%)
29%
Audited
FY14
16.1
5.4
34%
Pro forma Pro forma Pro forma
FY15
FY14
FY13
124.3
91.8
84.4
35%
9%
25.3
18.3
38%
2%
20%
20%
18.0
21%
Financial Year 2015
Significant sales growth, up 78%. On a proforma basis still excellent 35%
Very strong gross profit performance, up 53%, again strong on a proforma basis +38%
The healthy deal flow seen in FY15 has carried forward into FY16
Number of opportunities and value of the sales pipeline remains strong
A “Strategic Support Services” offering has been developed, helping larger pharma
companies take a more strategic approach to managing access to their portfolio globally.
The first real world data projects are expected to start in Q2 FY16, but discontinuation services
continue to be strong
© Copyright Clinigen Group plc. All rights reserved
Right Drug Right Patient Right Time
13
Idis Global Access
£m
12 months ended 30 June
Sales
Idis GA
Audited
FY15
9.2
Audited
FY14
-
YoY Growth %
Gross Profit
2.8
-
YoY Growth %
Gross Profit Margin (%)
30%
-
Pro forma Pro forma Pro forma
FY15
FY14
FY13
61.0
66.4
70.9
-8%
-6%
14.6
13.3
10%
-20%
24%
20%
16.7
24%
Financial Year 2015
Sales declining year on year as described in acquisition roadshow, due to changes in UK
“specials” market and one poor performing low margin commercial supply agreement
Decline in sales will continue in FY16 as commercial contract (+£25m sales) is closed
Strong gross profit performance, up 10%, following a period of decline related to loss of
UK Specials contracts in 2013/14
© Copyright Clinigen Group plc. All rights reserved
Right Drug Right Patient Right Time
14
Access across the lifecycle
Access across the lifecycle
Unlicensed
Licensed
Specialty
Pharmaceuticals
No approval
Ex-trial
Clinigen Clinical
Trial Services
Pre-launch
Phased
launch
Phase II
Phase III
EARLY
Pre-Approval
Phased Launch
EXPANDED
Market Exit
MATURE
Idis GA and Link together will help Clinigen meet the huge opportunity to supply the
global unmet for ethical medicines, estimated to be up to $7bn annually
Marketing the services globally to hospital and regional pharmacist and investment in
the e-commerce platform to reach the global market will be a feature of FY16 and FY17
© Copyright Clinigen Group plc. All rights reserved
Right Drug Right Patient Right Time
15
Clinigen Specialty
Pharmaceuticals
£m
Clinigen SP
Audited
FY15
33.7
Audited
FY14
26.9
Audited
FY13
24.3
YoY Growth %
25%
11%
12%
Gross Profit
29.1
23.2
19.8
YoY Growth %
25%
17%
8%
Gross Profit Margin (%)
86%
86%
82%
12 months ended 30 June
Sales
Audited
FY12
21.7
18.3
84%
Financial Year 2015
Sales and gross profit both showing 25% growth
SP remains the largest contributor to the Group’s gross profit accounting for 54%, 34% in the
new enlarges group on a proforma basis
Foscavir “risk” diluted by new products to 70% of SP sales and profit, significantly reduced from
86% at the end of FY14.
Ethyol acquired August 2014
Completed the final transfer of market authorisations for Cardioxane, Savene and Ethyol
Strategic Alliance with Cumberland Pharmaceuticals will provide support for Clinigen
products in the US, with Clinigen supporting Cumberland outside the US
© Copyright Clinigen Group plc. All rights reserved
DrugPatient
Right Patient
Right
Time
Right DrugRight
Right
Right
Time
16
Clinigen Specialty
Pharmaceuticals
Dexrazoxane – Savene and Cardioxane
All marketing authorisations (MA) and technical transfers completed
Savene sales ahead of those achieved by previous MA holder
Strong KOL support for Clinigen’s strategy of challenging Article 31 restrictions, from the worldrenowned Children’s Oncology Group (COG), with the paper co-authored by 25 global Key Opinion
Leaders. We anticipate a response from the regulators in the next six months
Ethyol
Rapid steps made in the revitalisation of Ethyol in FY15, transferring the MA for the US and Europe
Well underway with the technical transfer of the manufacturing, which will complete by end FY16
Good potential in the growth of Ethyol; new radiotherapy techniques still cause a reasonable
incidence xerostomia, which is untreated. No significant competitor currently in development
Foscavir
Growth reflects underlying disease it treats both growing at 4-5%
Launch in South Korea and US distribution agreement extended with Hospira through to end 2019
Supporting Japanese study to extend treatment into HHV6
Vibativ
Diagnostic e-Test continues to be a problem and although reimbursement agreed in UK, Ireland,
Germany and Austria, reimbursement has been refused in a number of other European markets
The e-Test, inconsistent reimbursement and current loss making position due to regulatory
demands has led to a review of its commercial viability in Europe
© Copyright Clinigen Group plc. All rights reserved
DrugPatient
Right Patient
Right
Time
Right DrugRight
Right
Right
Time
17
15
Key Strategic Objectives
The Clinigen Model
Only 3 routes to get a drug into a
patient, clinical trial, as a licensed
or unlicensed medicine. Clinigen
uniquely supplies all three
But;
80% of the world’s population, some
5.5bn people, have low or non-existent
access to medicines
An estimated 50% of drugs sold on-line
are fake
The proportion of counterfeit or substandard medicines can be as high 1030% in some regions
This is Global Healthcare Crisis!
A healthcare professional has to know the drug they want to treat their patient is; available (right
drug) and can be imported to his/her hospital (right patient) quickly (right time), this is the Clinigen
model
© Copyright Clinigen Group plc. All rights reserved
Right Drug Right Patient Right Time
19
Strengthening The Clinigen Model
To strengthen the right drug, right
patient, right time model, Clinigen
has:
Acquired Idis, giving it market
leader status in unlicensed and
Clinical Trial drug supply
Formed a strategic alliance with
Cumberland Pharmaceuticals in the
US to better control its own
products
Acquired Link Healthcare to give it
a local footprint in Southern Africa,
Australia, New Zealand, Singapore,
Malaysia, Hong Kong and Japan
© Copyright Clinigen Group plc. All rights reserved
Right Drug Right Patient Right Time
20
Key Strategic Objectives
Utilise Clinigen products database to identify opportunities in top 50 pharma
SO 1: Acquisition of product
opportunities from top
50 pharma
Develop relationships within pharma to identify opportunities
Develop relationship with “acquisition brokers”
Develop global distribution relationships and capabilities
Target is 5 products over next 5 years
Maintain Foscavir – new markets, South Korea. New indication
SO 2: Revitalize acquired assets
Dexrazoxane strategy to reverse article 31
Ethyol growth strategy
Strengthen sourcing capabilities for CTS and unlicensed medicines
SO 3: Extend global capabilities
Develop “international pharmacy” type unlicensed supply model to pharmerging markets
Develop a better direct supply model to key pharmerging markets for licensed and unlicensed supply
Extend business with current customers
SO 4: #1 Global CTS Company
Target key pharma, CRO, re-packers who are not current customers
Develop exclusive sourcing agreements
Develop “smart” global sourcing capabilities
Extend business with current customers
SO 5: #1 Global MA Company
Target key pharma who are not current customers
Develop software to create unique offering
Develop “international pharmacy” type unlicensed supply model to pharmerging markets
SO 6: Corporate Acquisitions
© Copyright Clinigen Group plc. All rights reserved
Acquisition of companies that both strengthen Clinigen’s portfolio and/or global footprint and
distribution capabilities
Right Drug Right Patient Right Time
21
Strategic Progress
SO 4: #1 Global CTS Company
Consolidated global market leader position with acquisition of Idis
SO 5: #1 Global MA Company
The Idis acquisition allowed us to combine the Idis MAP and Clinigen GAP businesses
under the Idis MA brand, catapulting the Group into the global market leader
position in the exclusive managed access sector
SO 6: Corporate Acquisitions
Acquisition of companies that both strengthen Clinigen’s portfolio and/or global
footprint and distribution capabilities
© Copyright Clinigen Group plc. All rights reserved
Right Drug Right Patient Right Time
22
© Copyright Clinigen Group plc. All rights reserved
Right Drug Right Patient Right Time
23
Acquisition of Link Healthcare
© Copyright Clinigen Group plc. All rights reserved
Right Drug Right Patient Right Time
24
Overview of Acquisition
Clinigen is acquiring Link Healthcare, a specialist Pharmaceutical and Medical Technology business
focussed in the regions of Asia, Africa and Australasia, for an initial consideration of £44.5 million
The acquisition is for a maximum consideration of £100m on a debt-free cash-free basis, payable
in 3 tranches over 3 years, subject to performance:
£44.5m payable at completion, 50% in new shares and 50% in cash
Up to two further tranches, to a maximum of £55.5m in total, depending on the
achievement of EBITA for the financial years ending June 2016 and June 2017.
Initial cash element of the consideration will be financed from Clinigen’s existing debt
facilities
Will apply for listing of new share’s, the consideration will comprise 3,102,558 Ordinary
Shares, calculated based on the average share price over the 10 trading days ending 18th
September 2015, and will be admitted to trading on 30 October
The Acquisition is expected to be immediately earnings enhancing
Completion is expected to be 30 October, following the Clinigen AGM
© Copyright Clinigen Group plc. All rights reserved
Right Drug Right Patient Right Time
25
Overview of Link
Link was founded in 1997 and focussed in the regions of Asia (Singapore, Malaysia, Hong Kong & Japan),
Southern Africa and Australia, New Zealand, employing c.150 people
Link offers a range of services on a local or cross-regional basis:
Full commercial launch services
Mature and discontinued product lifecycle management
Comprehensive regulatory services
Marketing and logistics
Early market access programs, including the sourcing of “Named Patient based Supply” (NPS) for more
than 200 essential medicines
Link is the holder of over 100 Marketing Authorisations in its local markets
Has long-term partnerships with over 40 global blue chip pharma and medical technology clients
For the financial year ended 31 March 2015, Link achieved revenue of £31.6 million and EBITDA of £4.2 million.
It has gross assets of £23.3 million as at 31 March 2015. In the LTM period to June 2015 Link achieved revenue
of £33.7 million and EBITDA of £5.1 million.
Link’s mission is a commitment to excellence in the provision of vitally important specialty products and to
securing innovative medicines and technology, delivering access to world class therapies
© Copyright Clinigen Group plc. All rights reserved
Right Drug Right Patient Right Time
26
Strategic Benefits
The acquisition fulfils a number of Clinigen’s strategic ambitions and will increase future growth opportunities,
both in new markets and globally, and will support all areas of the business through:
A significant AAA (Asia, Africa & Australasia) footprint, with an established, profitable and trusted
business in key markets
Immediately growing the portfolio of products and services
Increasing distribution capabilities in line with our hub and spoke model
Providing registration and commercialisation capabilities in the region
The new combined entity will further strengthen our position as the global market leader in ethical unlicensed
supply - a large addressable and under-penetrated market with the opportunity to shape and develop a
$5+billion market
Providing regional on-demand unlicensed supply capabilities in established and ‘pharmerging’ markets
Strengthening key customer links with hospital pharmacists and KOL across the AAA region
Providing access to Link’s exclusive relationships for both licensed and unlicensed products
Providing complementary customer lists in unlicensed supply
Further accelerates Clinigen’s goal of market leader status
© Copyright Clinigen Group plc. All rights reserved
Right Drug Right Patient Right Time
27
Summary
Key Acquisition Highlights
1
Strategic rationale – significant step in achieving stated strategy to develop global footprint
2
Significantly expanded distribution and sales capability – opportunity to leverage market leadership as
Link has complementary relationships with pharma, biotech, health authorities and regulators
3
Direct access to fast growing pharmerging markets through existing established Link customers
4
Further enhances life cycle management service offering through local registration and commercialisation
in the pharmerging markets for discontinued, rare and orphan drugs
5
Established methodology for commercial supply agreements which can be extended
6
Earnings enhancing and cash generative
© Copyright Clinigen Group plc. All rights reserved
Right Drug Right Patient Right Time
28
Cumberland Pharma
Clinigen Group signs strategic alliance with
Cumberland Pharmaceuticals
Partnership strengthens Clinigen’s global footprint
and gives Cumberland Pharmaceuticals access to
new international markets
Cumberland Pharmaceuticals acquires, develops and commercializes branded
prescription products designed to improve quality of care and address unmet medical
needs. The company focuses on underserved niche markets in the US and currently
markets five FDA approved products. These products include Acetadote® (acetylcysteine)
injection for the treatment of acetaminophen poisoning and Vaprisol® (conivaptan)
injection, for the treatment of hyponatremia. Cumberland also has a development
pipeline with candidates in Phase II studies.
© Copyright Clinigen Group plc. All rights reserved
Right Drug Right Patient Right Time
29
Next Steps for the Enlarged Group
Integration of acquisitions
Idis; focus on commercial synergies and e-commerce platform
Link; start 100 day integration plan
Cumberland; map out product opportunities
Launch of new added value services in MA and CTS and continue revitalisation of products
The Board remains confident in the outlook for the full year
This has been a transformational year
whilst delivering strong financial performance
© Copyright Clinigen Group plc. All rights reserved
Right Drug Right Patient Right Time
30
Appendices
Senior Management
Peter George – Chief Executive Officer
Joined Clinigen when it formed in June 2010
Former CEO at Penn Pharma, having led a GBP67m management buy-out (2007)
Previously executive VP for Wolters Kluwer Health with responsibility for Europe/Asia Pacific
Former Chief Operating Officer of Unilabs Clinical Trials International Limited
Shaun Chilton – Deputy CEO
Joined Clinigen in January 2012
Previously President within KnowledgePoint360 Group, a global pharmaceutical information and
services operation
20 years’ commercial, strategic and operational experience, including sales and marketing in Pfizer
and Sanofi-Aventis (now Sanofi)
Robin Sibson – Chief Financial Officer
Joined ADL Healthcare Limited, a company owned by Clinigen’s former Executive Chairman (2003)
Over 30 years’ experience in the pharmaceutical industry, including 15 years as Finance Director
Formerly Finance Director of BASF’s UK Pharmaceuticals, sales and R&D divisions
Previously Finance Director at Boots UK Pharmaceutical business, leading the integration
following its sale to BASF
Martin Abell – Chief Financial Officer Elect
• Martin joined Clinigen in August 2015 as CFO Elect.
• Previously at the FTSE250 recruitment group Hays plc. as Head of Investor Relations and M&A,
and most recently as Finance Director for the Continental Europe and Rest of World division
• Prior to Hays Martin held financial roles in Exel plc (now part of Deutsche Post)
• Qualified Chartered Accountant, having trained at PwC in the M&A Transaction Services team.
© Copyright Clinigen Group plc. All rights reserved
Right Drug Right Patient Right Time
32
Clinigen Specialty
Pharmaceuticals
Dexrazoxane
Cardioxane acquired from Novartis in 2013, used for as a cardioprotective drug against
anthracycline toxicity and Savene acquired from Norgine in 2014, used to treat extravasation
caused by anthracycline treatment
Ethyol
Acquired from AstraZeneca August 2014, it is a cytoprotective drug indicated to reduce the
incidence of xerostomia (dry mouth) in patients undergoing radiation treatment for head and
neck cancer and to reduce renal toxicity associated with cisplatin in patients with advanced
ovarian cancer
Foscavir
Acquired global rights from AZ in 2010; Anti-viral last line treatment predominantly used for
CMV in bone marrow transplant patients
Vibativ
In-licensed Vibativ from Theravance in 2013, used to treat hospital acquired pneumonia of an
MRSA cause
© Copyright Clinigen Group plc. All rights reserved
DrugPatient
Right Patient
Right
Time
Right DrugRight
Right
Right
Time
33
15