Pageflex Server [document: D-0041-00035393_00001]
advertisement
![Pageflex Server [document: D-0041-00035393_00001]](http://s2.studylib.net/store/data/018579708_1-fbca673435f8f2686fdbc98d5b53d510-768x994.png)
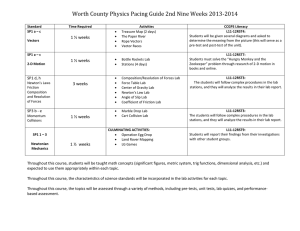
CONFIRM Estrogen Receptor (ER) (SP1) Rabbit Monoclonal Primary Antibody Breast carcinoma ER positive / Magnification: 40X Empowering clinical confidence • Rabbit Monoclonal Primary Antibody • Pre-diluted, ready-to-use reagent • Fully automated on Benchmark IHC/ISH staining platforms • CE-IVD marked/FDA 510(k) cleared Accurately identify patients for hormone receptor targeted therapy Estrogen receptor (ER) is a powerful predictor of the response to hormone therapy (such as Tamoxifen) and clinical outcome of breast cancer patients. Run on Ventana BenchMark IHC/ISH automated slide staining instruments with our advanced detection chemistry, the CONFIRM ER (SP1) Rabbit Monoclonal Primary Antibody can deliver: • More precise determination of ER expression status compared to other on-market clones and assays to ensure eligible patients are identified for hormone therapy1,2,3,10 • Superior sensitivity and specificity in breast carcinoma samples compared to mouse monoclonal antibodies4,10 • Faster, more accurate results with complete automation and ready-to-use reagents5 CONFIRM ER (SP1) is shown to have a high concordance with the ER (6F11) antibody (96% concordance) but demonstrates a superior signal-to-noise ratio. This is particularly important in low-expressing ER+ cases. Difficulties with the interpretation of clone 6F11 staining patterns due to weak granular/punctate nuclear staining can lead to falsepositive results.1, 6 HER-2/neu (4B5) HER-2 Dual ISH PR (1E2) ER (SP1) Ki-67 (30-9) CONFIRM ER (SP1) is part of the Ventana Breast Cancer Diagnostics solution. References 1. Tubbs R, et al. Clinical utility of the CONFIRM anti-Estrogen Receptor (SP1) Rabbit Monoclonal Antibody. White Paper. Ventana Medical Systems, Inc. Tucson, Arizona. May 2012. 2. Cheang M, et al. Immunohistochemical Detection Using the New Rabbit Monoclonal Antibody SP1 of Estrogen Receptor in Breast Cancer Is Superior to Mouse Monoclonal Antibody 1D5 in Predicting Survival. JCO. 2006; 24(36): 5637-5644. 3. Welsh A, et al. Quantitative Analysis of Estrogen Receptor Expression Shows SP1 Antibody Is More Sensitive Than 1D5. Appl Immunohistochem Mol Morphol. 2012. [Published online ahead of print July 19 2012]. DOI: 10.1097/PAI.0b013e31825d73b2. 4. Rossi S, et al. Rabbit monoclonal antibodies: a comparative study between a novel category of immunoreagents and the corresponding mouse monoclonal antibodies. Am J Clin Pathol. 2005; 124(2): 295-302. 5. Assessment Run B13 2012. Estrogen receptor (ER). NordiQC. http://www.nordiqc.org/Run-35-B13-H1/ Assessment/assessment-B13-ER.htm. Updated 10 JUL 2012. Accessed 28 AUG 2012. 6. Rakha E, et al. Low–Estrogen Receptor–Positive Breast Cancer: The Impact of Tissue Sampling, Choice of Antibody, and Molecular Subtyping. JCO. 2012; 30(23): 2929-2930. 7. BenchMark XT IHC/ISH Automated Slide Preparation Systems [Operator’s Manual]. Tucson, AZ: Ventana Medical Systems, Inc.; 2010. 8. BenchMark ULTRA IHC/ISH Automated Slide Preparation Systems [Operator’s Manual]. Tucson, AZ: Ventana Medical Systems, Inc.; 2011. 9. CONFIRM anti-Estrogen Receptor (ER) (SP1) Rabbit Monoclonal Primary Antibody [package insert]. Tucson, AZ: Ventana MedicalSystems, Inc.; 2013. 10.Bae et al. Hormone Receptor Expression in Invasive Breast Cancer Among Korean Women and Comparison of 3 Antiestrogen Receptor Antibodies: A Multi-institutional Retrospective Study Using Tissue Microarrays. Am J Surg Pathol. 2012; 36(12):1817–1825. CONFIRM ER (SP1) delivers on three key benefits that are valued by pathology professionals: Clinical superiority SP1 more accurately identifies breast cancer patients for hormone therapy: • Cheang et al. conclude 1D5 failed to identify 6% of ER-positive patients compared to SP1, and that SP1 is 8% more sensitive, demonstrating that “SP1 represents an improved standard for ER immunohistochemistry assessment in breast cancer.” 2 • Welsh et al. conclude that “SP1 is more sensitive than 1D5, and displays a stronger signal-to-background ratio. This would suggest that, in addition to its benefits for cost efficiency, the use of SP1 in a clinical setting may help reduce the false-negative rate.”3 • Bae et al. demonstrate that ER(SP1) was more sensitive in identifying ER expression in tumours than the mouse monoclonal clones 1D5 and 6F11 as supported by positive correlation to overall survival.10 Analytical superiority Specific and sensitive pre-diluted rabbit monoclonal antibodies help you diagnose precisely and confidently: • Ready-to-use reagents, like CONFIRM ER (SP1), consistently demonstrate superior performance when compared to concentrated antibodies that are used in in-house validated assays.5 • Rossi et al. conclude through a comparison of RbMAbs against the corresponding MMAbs that RbMAbs clearly demonstrated a significant increase in terms of sensitivity4 • CONFIRM ER (SP1) is shown to have a high concordance with the ER (6F11) antibody (96% concordance) but demonstrates a superior signal-to-noise ratio.1 This is particularly important in low-expressing ER+ cases. Difficulties with the interpretation of 6F11 staining patterns due to weak granular/punctate nuclear staining may lead to false-positive results. 6 Testing efficiency Rapid and consistent results delivered through fully automated platforms, ready to use reagents and digital pathology: • Protocols on BenchMark IHC/ISH staining platforms show time to result of ~ 3.5 hrs 7, 8 • Product development verification and validation studies show reproducibility for inter and intra-platform, lab and reader 9 • Maximise workflow efficiency with digital pathology CONFIRM ER (SP1) Ordering information Product name Catalogue number Order number Quantity CONFIRM anti-Estrogen Receptor (ER) (SP1) Rabbit Monoclonal Primary Antibody 790-4324 05278406001 50 tests CONFIRM anti-Estrogen Receptor (ER) (SP1) Rabbit Monoclonal Primary Antibody 790-4325 05278414001 250 tests Roche Diagnostics (Schweiz) AG Industriestrasse 7 CH-6343 Rotkreuz Switzerland Tel: +41 (0)41 799 61 00 Fax: +41 (0)41 799 65 45 www.roche.com www.ventana.com © 2013 Ventana Medical Systems, Inc. VENTANA, the VENTANA logo, BENCHMARK and CONFIRM are trademarks of Roche. All other trademarks are the property of their respective owners. E536 0113E 06509428001 VENTANA Empowering | Clinical Confidence


![Anti-IL12RB1 antibody [MM0363-7V24] ab89890 Product datasheet Overview Product name](http://s2.studylib.net/store/data/012509392_1-c59a48d95428df03c00f8fd937d26c93-300x300.png)