Solubility Product Constant Lab: PhET Simulation
advertisement

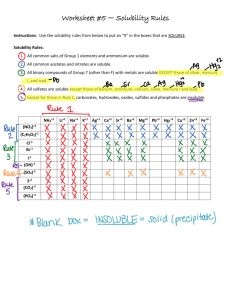
Name: Date: Lab 26: PhET: The Solubility Product Constant Accelerated Chemistry Go to the following website for the PhET : http://phet.colorado.edu/en/simulation/soluble-salts Click “Run Now” to the launch the simulation. You may have to click the arrow in the upper right-hand corner of the screen and select the “soluble salts” download. When Java launches, click “Run.” Part 1. Solubility of Table Salt A screen will open that looks like the one pictured to the right. You should see a tab that says “Table Salt.” This is the compound that will come out of the salt-shaker when you “shake it.” Answer the following questions: 1. Write the equation for sodium chloride dissolving in water. 2. Predict what you will see when you add the salt to the water in the simulation. 3. If you were dissolving table salt in a beaker of water, what would a saturated solution look like? Use the simulation to see what a saturated solution looks like on a microscopic level. Talk about how you know a solution is saturated. Check with Mrs. Giammanco to see if your reasoning is correct! Write a description of a saturated salt solution microscopically and macroscopically, and support your ideas with drawings. 4. Calculate the solubility of a saturated solution of sodium chloride in molarity. (Hint: What are the number of dissolved Na+ ions? What is the mole ratio of NaCl to dissolved Na+ ions? How would you covert # of dissolved ions to moles?) Lab26-KspPHET 1 Part 2. Solubility of “Insoluble Salts” Click on the tab that says “Slightly Soluble Salts.” Begin with the salt mercury (II) bromide. This can be selected from the drop-down tab at the upper right-hand side of the screen. 1. Based upon your solubility rules, should mercury (II) bromide be soluble or insoluble? Explain. 2. Write an equation for mercury (II) bromide dissolving in water. 3. Shake some of the mercury (II) bromide salt into the water. Describe the solution. How does this compare with the table salt solution from the previous section? 4. Calculate the solubility of a saturated solution of mercury (II) bromide in molarity. How does this compare with the sodium chloride solution from the previous section? 5. Select strontium phosphate from the drop-down tab in the upper right-hand corner of the screen. Based upon your solubility rules, should strontium phosphate be soluble or insoluble? Explain. 6. Write an equation for strontium phosphate dissolving in water. Lab26-KspPHET 2 7. Shake some of the strontium phosphate salt into the water. Describe the solution. 8. Calculate the solubility of a saturated solution of strontium phosphate in molarity. 9. Explain in your own words what it means for a salt to be classified as “insoluble.” 10. Is dissolving a soluble salt an equilibrium process? Is dissolving an insoluble salt an equilibrium process? Explain your thoughts. 11. If dissolving is an equilibrium process, what would be the equilibrium constant expression for dissolving mercury (II) bromide in water? (Hint: Look at the equation you wrote for dissolving it in water.) 12. Using the solubility data from question 4, calculate the equilibrium constant for the dissolution of mercury (II) bromide. Lab26-KspPHET 3