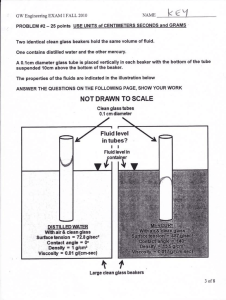
Lecture, Thursday May 21, 2015 Physics 105C
advertisement

Lecture, Thursday May 21, 2015 Physics 105C The key equations we have so far governing fluid flow, each of which comes from a particular conservation law, are: ∂ρ + ρ∇ · v = 0 (continuity; from mass conservation) ∂t ∂v + (∇v) · v = ρB − ∇p + µ∇2 v (Navier-Stokes; from momentum conservation) ∂t + (∇Θ) · v) = 2µT r(DD) + κ∇2 Θ (heat equation; from energy conservation) ρc( ∂Θ ∂t The continuity equation is general, but as written the other two apply only to incompressible fluids. Additional terms including volume changes would have to be added for compressible fluids. As written, these equations are a coupled system that determine v, p, and Θ. These are one vector and two scalar quantities, so one vector equation (Navier-Stokes) and two scalar equations is appropriate. As noted last time, velocity comes into the heat equation through D, but temperature does not appear in the Navier-Stokes equation. Hence the system can be solved by finding velocity and pressure from the first two equations, then using that velocity field to find the tempearture Θ. If it feels strange that the interplay between temperature and velocity goes in one directiononly, that’s because in most physical systems there is indeed influence in both directions. In the Navier-Stokes equation, the density ρ (and also the viscosity µ) typically depend on temperature. We’ll ignore any such dependence of µ, but we’ll look at how temperature-dependence of ρ affects the solutions. The density will be yet another field, varying with position and time. Adding this extra variable means we need yet another differential equation. What’s used is an equation relating ρ to Θ, and possibly to p. One possibility is the ideal gas law, P = RρΘ. A different, frequently-used fourth equation is a statement that the 0 = −α(Θ−Θ0 ). The relative change in density is proportional to the change in temperature: ρ−ρ ρ0 minus sign on the left allows α to be positive in the usual situation that increasing temperature decreases density. This second option is a particularly simple dependence. The lack of pressuredependence means that at any fixed temperature the fluid is incompressible. Furthermore, as long as temperature changes are “small,” then a Taylor expansion gives exactly this linear dependence. Hence it can be a realistic approximation in various physical situations. Unfortunately, even in extremely simple geometries the combined set of four equations is essentially impossible to solve. As mentioned earlier, the nastiness in the incompressible NavierStokes equation comes from the non-linear velocity gradient term. We were able to find exact solutions to laminar flow in some very simple cases where that term vanished. (But we’ll come back to it next week when we talk about instabilities that lead to turbulence.) Now that ρ is also one of the coupled variables, there are all manner of non-linear terms – and on top of that, the relationship between ρ, Θ, and p may itself be non-linear. As a result, most of the rest of the course will involve qualitative or at best semi-quantitative discussion. There are three mechanisms for heat transfer. One is radiative transfer, where an object emits photons that are absorbed by another object. If the objects act as black bodies, then the Stefan-Boltzmann law governs the radiation: the amount of energy radiated per time, per unit surface area, is σΘ4 . This is often a reasonable approximation. Even when it doesn’t hold, the correction is often to multiply by a constant (the emissivity) between 0 and 1; this is called a ”grey body.” Given two objects at temperatures Θ1 and Θ2 , the radiative heat transfer between them is qrad = σ(Θ41 − Θ42 ). The radiation term is immensely complicated in practice (imagine a fluid divided into small elements, all at different temperatures), but fortunately it’s typically only important for extremely high temperatures or very large temperature differences, so we will simply ignore it. The second heat transfer mechanism is conduction, described by qcond = −κ∆Θ. This was the relevant mechanism last time in deriving the temperature profile within a flow. The picture behind conduction is that the atoms within a fluid collide with each other, and these collisions transfer heat. The heat then travels across the material, although individual atoms may not travel far from their original locations. The third mechanism is convection, in which fluid particles transport heat by moving from one region to another. Flow from a hot region to a cold one delivers energy, while flow from cold to hot absorbs energy from the hot region. In “natural” or “free” convection, the fluid flow is set up through the existence of hot and cold boundaries (or immersed objects), sometimes along with gravity. In “forced” convection, the flow is established through stirring or a drive such as pressure. The classic example of convection is heating a pot of liquid on a stove burner. Before turning on the heat, gravity establishes a pressure gradient. The heat enters at the bottom, warming the liquid there. That makes the liquid expand (ρ decreases), and it then moves upwards under the influence of gravity so that higher-density fluid can take its place at the bottom. (This is the gravitationally stable arrangement, just as oil and water separate .) As the hot fluid rises, it moves farther from the heat source, cools down, becomes more dense, and sinks downward again in its turn. The entire process is a circular motion, with fluid moving upward in some parts of the pot and downward in others. Another example of heat transfer, which involves convection but not a convection loop, appears in Taconis oscillations. This phenomenon occurs in a narrow tube with its ends at very different temperatures. The hot end should be closed and the bottom end open. In particular, say that the top end is at room temperature (300 K) while the bottom end is in a dewar of liquid helium at 4 K. When the tube is inserted into the dewar, the gas at the bottom cools and gets denser. The bottom end is open, so additional gas or liquid can be drawn in from the dewar. Gas is also pulled downward from the upper reaches of the tube, setting up a larger pressure gradient than happens with gravity alone at a constant temperature. The low pressure at the upper end of the tube begins to pull gas upward, compressing the gas at the hot end of the tube. One might think that things would eventually settle down, with a steady pressure gradient that’s larger than what it would be due solely to gravity. If the temperature gradient is small enough, that’s exactly what happens. However, a sufficiently large temperature gradient causes overshoot. As cold gas moves upward, it warms up from the compression. (The simple picture is that the compression is adiabatic, or reversible. This requires a particular relationship among presure, density, and temperature.) If the temperature along the tube changes quickly enough, the gas nonetheless encounters tube walls that are even warmer than it is. It gains additional energy through heat exchange with these walls. (The walls add yet another variable to the set of governing equations. Heat conduction from the wall to the fluid is qinterf ace = h(Θwall − Θf luid ), where the heat transfer coefficient h has nothing whatsoever to do with Planck’s constant in quantum mechanics!) This means that eventually, when the density increase hits the closed top of the container, it has overshot and the gas must move back down. Similarly on the way down, downward moving gas cools by expansion but also cools through heat exchange with the even colder walls it encounters along the way. (See http://web.mit.edu/8.13/www/pdf files/taconisoscillations-experimental-cryophysics-hoare-1961.pdf for a slightly more detailed description.) Taconis oscillations are both used and avoided in low-temperature physics. In early work by Taconis they were used to stir helium. The common use now is to detect the level of liquid helium within a dewar. The frequency (and amplitude) of the Taconis oscillations depend on whether the cold end of the tube is open to gas or immersed in liquid. It’s easy to monitor the oscillations by using a flexible membrane to close off the top end (e.g., tying part of a rubber glove over the opening). Then the user adjusts to the level at which the frequency and amplitude abruptly change. The less pleasant news about Taconis oscillations is that they carry energy from room temperature into the helium dewar: the helium gas in the tube absorbs energy from the hot walls, and subsequently deposits energy into the lower, colder walls. The energy transfer is partly through convection and partly from conduction. Note that there’s never a convective loop such as having gas move up along the walls and down in the middle of the tube; rather, the gas travels up for a while and subsequently reverses direction and travels down. The energy transfered to the liquid helium can be 1000 times larger than that from conduction along the tube in the absence of oscillations. This is catastrophically expensive. (My lab sometimes buts a dewar with 250 liters of liquid helium, which we use for about one month. At 1000 times the boiloff rate, it would instead last about one hour!) On cryostats designed for use with liquid helium, all tubes (magnet leads, shields for wiring, support structures) that might produce Taconis oscillations have a few holes drilled along the side to prevent the oscillations from developing. There has been some work at Los Alamos to develop this type of thermoacoustic cycle into a refrigeration technique. It could have some advantages, notably no moving parts. These oscillations were originally discovered in an entirely different situation: glassblowing, where room temperature is the low temperature end. A glassblower blows through an open tube; the piece being worked on seals the opposite, high-temperature end of the tube. Glassblowers noticed the oscillation as a whistling sound. Quite a few conditions need to be met for selfsustaining oscillations. One is that for reasonable tube sizes, the temperature ratio at the two ends should be about 6. In fact Taconis oscillations are usually not observed with liquid nitrogen (ratio 4 from room temperature) but appear easily in liquid helium (ratio 75). The contact between the gas and the wall can’t be too good, or the gas will simply track the wall temperature exactly on both the way up and the way down, and it won’t transfer energy from one part of the wall to another. The gas-wall contact also can’t be too weak, or the gas will effectively be isolated in a temperature-independent environment. Quantitative numerical work calculating the oscillation frequency and amplitude continues. Even this relative simple geometry of a straight cylinder with a temperature gradient along it requires various simplifying assumptions, such as ignoring any axial (radial) motion and temperature-dependence. Clearly the latter is not entirely realistic; the gas at the outer edge of the tube responds to the temperature of the tube surface, but the temperature information must then work towards the tube center through conduction. We’ll now turn to additional simplifications on fluid flow, looking at irrotational flow (∇×v = 0 in an inviscid (µ = 0), incompressible (∇ · v = 0) fluid. Superfluid helium is a marvelous example, but this can even give insight into classical fluids. The biggest issue for classical fluids is the irrotational assumption. For superfluids the main issue is the zero viscosity assumption, since at non-zero temperature the superfluid is coupled to a normal fluid with non-zero viscosity. In any case, for an inviscid incompressible fluid the stress tensor becomes Tij = −pδij , and the Navierdp i = − dx + ρBi , which is known as the Euler equation. In both of Stokes equation becomes ρ Dv Dt i these the simplification comes from dropping a term proportional to µ. The Euler equation is in Cartesian coordinates. At first glance the Euler equation looks linear, but that’s just because I wrote the left side as the material derivative of velocity. The nonlinear velocity gradient term is still present, just hidden within the material derivative. Can we find an irrotational solution to the Euler equation? Well, if ∇ × B 6= 0, then definitely not. For an irrotational solution, the curl of the left side would vanish (after swapping various pairs of derivatives so that the curl acts directly on the velocity), and on the right side ∇ × ∇p = 0 since the curl of a gradient always vanishes. That leaves nothing to balance the curl of B. However, as we’ll see next time, if B is conservative (which is equivalent to ∇ × B = 0, which is equivalent to the integral of B around any closed loop being zero, which is equivalent to the existence of a function Ω with B = −∇Ω), then an irrotational solution for v does always exist.