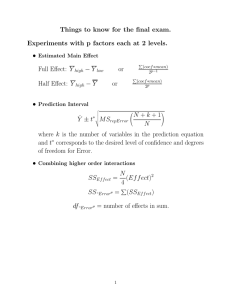
Electroconvulsive therapy for depression (Protocol)
advertisement

Electroconvulsive therapy for depression (Protocol) Leiknes KA, Berg RC, Smedslund G, Jarosch-von Schweder L, Øverland S, Hammerstrøm KT, Høie B This is a reprint of a Cochrane protocol, prepared and maintained by The Cochrane Collaboration and published in The Cochrane Library 2011, Issue 5 http://www.thecochranelibrary.com Electroconvulsive therapy for depression (Protocol) Copyright © 2011 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd. TABLE OF CONTENTS HEADER . . . . . . . . . . ABSTRACT . . . . . . . . . Figure 1. . . . . . . . . BACKGROUND . . . . . . . OBJECTIVES . . . . . . . . Figure 1. . . . . . . . . METHODS . . . . . . . . . ACKNOWLEDGEMENTS . . . REFERENCES . . . . . . . . APPENDICES . . . . . . . . HISTORY . . . . . . . . . . CONTRIBUTIONS OF AUTHORS DECLARATIONS OF INTEREST . SOURCES OF SUPPORT . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Electroconvulsive therapy for depression (Protocol) Copyright © 2011 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1 1 2 4 5 6 7 14 14 18 28 28 28 28 i [Intervention Protocol] Electroconvulsive therapy for depression Kari A Leiknes1 , Rigmor C Berg1 , Geir Smedslund1 , Lindy Jarosch-von Schweder2 , Simon Øverland3 , Karianne T Hammerstrøm1 , Bjørg Høie1 1 Norwegian Knowledge Centre for the Health Services, Oslo, Norway. 2 Department of Research and Development, St. Olavs University Hospital, NTNU-Faculty of Medicine, Department of Neuroscience, Trondheim, Norway. 3 Department for Health Promotion and Development, University of Bergen, Bergen, Norway Contact address: Kari A Leiknes, Norwegian Knowledge Centre for the Health Services, Postboks 7004, St. Olavs plass, Oslo, N-0130, Norway. KariAnn.Leiknes@Kunnskapssenteret.no. Editorial group: Cochrane Depression, Anxiety and Neurosis Group. Publication status and date: New, published in Issue 5, 2011. Citation: Leiknes KA, Berg RC, Smedslund G, Jarosch-von Schweder L, Øverland S, Hammerstrøm KT, Høie B. Electroconvulsive therapy for depression. Cochrane Database of Systematic Reviews 2011, Issue 5. Art. No.: CD009105. DOI: 10.1002/14651858.CD009105. Copyright © 2011 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd. ABSTRACT This is the protocol for a review and there is no abstract. The objectives are as follows: To assess the effects (benefits and harms) of ECT for depression in adults - and to integrate patient-lived experiences of ECT given for depression into the effectiveness review by novel methodology. (See sub-heading “The Integrative Methodological Approach” ). We will assess effects of ECT on a short- (immediately post treatment up to six months) and long-term (six months or more posttreatment commencement) basis, with special focus on long-term cognitive (memory) impairment. The review questions are: 1. Effectiveness 1.1 Effectiveness (benefits) How effective is ECT, on a short- and long-term basis, at relieving the symptoms of depression and at promoting full recovery of cognitive functioning (memory) and social functioning (activities of daily life) compared to other common treatments for depression (e.g. psychotherapy/cognitive behavioral therapy (CBT), antidepressants)? • Are there specific gender differences* and/or sub-groups of patients, such as voluntary versus involuntary*, treatment-resistant or psychotic forms of depressive illness, who respond better to ECT compared to other treatments? • Are there differences in the efficacy of various forms of ECT (electrode placement, dose, waveform and frequency)? • Is the effect of ECT sustainable over time? • Is there evidence to support the use of continuation/maintenance ECT? 1.2 Adverse effects (harm) How harmful is ECT, on both a short- and long-term basis, especially with regards to inducing transient or permanent cognitive impairment? Electroconvulsive therapy for depression (Protocol) Copyright © 2011 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd. 1 • Are the adverse effects (i.e. neuropsychological impairment) of ECT different for the different forms (e.g. bilateral, unilateral or high-dose, low-dose) of ECT? • Are there differences in the rates of adverse events secondary to various forms of ECT, e.g. bilateral versus right-unilateral, highdose v. low-dose? • What are the direct risks (i.e. harms*) of ECT (e.g. grand mal seizure), which could be experienced at the time of the actual procedure, including during the administration of anaesthetic? 2. Patient experiences What is the lived experience of ECT and how do patients perceive the effect of ECT as a therapy? The Integrative Methodological Approach We will conduct a systematic review which integrates qualitative evidence of the individual patient’s experience of the intervention (i.e. ECT as a treatment for depressive illness) into the effectiveness review. The approach was developed by the review team specifically for this systematic review question and was informed by Chapter 20 of the Cochrane Handbook of Systematic Reviews of Interventions (Noyes 2008). We will use qualitative data in parallel syntheses and juxtaposed alongside quantitative data, in order to i) inform and ii) extend the effectiveness review, as defined in Chapter 20 of the Handbook. The integrative four-phase methodological approach that we will undertake in this review is described in further detail in Appendix 1 and illustrated in Figure 1. Figure 1. The Integrative Methodological Approach We will treat qualitative and quantitative evidence as two separate streams during the data extraction process: 1. Effectiveness (benefits and harm) and 2. Patient experiences. However, in phase four in order to aid the interpretation of synthesized quantitative evidence we will juxtapose the qualitative evidence alongside it. Electroconvulsive therapy for depression (Protocol) Copyright © 2011 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd. 2 We will mark text added during phase two (refinement of the protocol) with an asterisk (*) in the revised protocol. Results from the qualitative evidence aiming to i) inform the effectiveness review by refining the protocol’s research questions and population, intervention, comparison and outcome (PICO) elements are given in Appendix 2. This is the revised version of the protocol, and we have undertaken the protocol refinement based on the qualitative evidence in two main areas: population (P) and outcome (O). Electroconvulsive therapy for depression (Protocol) Copyright © 2011 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd. 3 BACKGROUND Electroconvulsive therapy (ECT) has been used in varied dose ranges and schedules for at least the past 70 years in the treatment of a range of mood disorders, schizophrenia and some non-psychiatric medical conditions (UK ECT RG 2003). Clinical experience has long indicated that ECT can be effective in depressive illness (McCall 2001). Following the introduction of effective antidepressant pharmacotherapy, ECT practice radically changed. The use of ECT declined considerably in the 1970s and 1980s, and the indication for ECT also transformed from first-line treatment of depression to last-resort treatment for pharmacotherapy-resistant depression and very severe life-threatening clinical conditions (Eranti 2003; McCall 2001). Worldwide, one million patients are estimated to receive ECT annually (Prudic 2001), and it is still frequently used to treat depressed elderly (Stek 2003). However the use of ECT for depression varies greatly (Chanpattana 2005; Chanpattana 2007; Chanpattana 2010; Eranti 2003; Gazdag 2009; Ikeji 1999; Little 2003; Nelson 2005; Okagbue 2008; Sienaert 2005). Scientific literature, and ECT guidance handbooks by professional organizations and regulatory bodies (Abrams 2002; APA 2001; CPA 2001; Fink 1999) report an overall effectiveness of ECT for depression. However, although benefits are perceived by patients (Malekian 2009; Rajkumar 2007), ECT is still a treatment form regarded by many as controversial, stigmatizing, and potentially associated with severe adverse effects on memory (Carr 2007; Fraser 2008; Ingram 2008; Rose 2003; Sackeim 2007; Sterling 2000). Consumer views (Carr 2007; Koopowitz 2003; Rose 2003) differ considerably from the mainstream published literature from regulatory bodies on the benefits of ECT (APA 2001; NICE 2003; RCP 2005; UK ECT RG 2003). A systematic review and meta-analysis from 2003 (UK ECT RG 2003) indicated there may be a trade-off between making ECT optimally effective and limiting the cognitive impairment. However, long-term effects of ECT on cognitive function are largely unknown. Although conventional magnetic resonance imaging (MRI) has failed to reveal structural brain changes after ECT, newer techniques such as diffusion weighted imaging (DWI) have revealed possible changes in the hippocampus formation area of the brain (Szabo 2007). Description of the condition Characteristic symptoms of a major depressive episode include a period of at least two weeks of depressed mood or the loss of interest or pleasure in nearly all activities of daily life. In addition to the symptoms being persistent most of the day, today’s current diagnostic criteria (APA 2000; WHO 1993) require the presence of at least four other symptoms from a list, such as feelings of worthlessness or guilt, difficulty thinking or concentrating, recurrent thoughts of death or suicidal ideation, suicidal plans or attempts and changes in appetite or weight or psychomotor activity. Severity of the depressive illness is given in terms of being mild, moderate or severe, in addition to other characteristics or specifiers, such as with or without psychotic (i.e. hallucinations, delusions) features. Depression is a common disorder, affecting about 151 million people worldwide (WHO 2008). The burden of depression is 50% higher for women than men, and mental disorders (including depression) are among the 20 global leading causes of disability (WHO 2008). Lifetime prevalence of major depressive disorder (MDD) examined in 10 countries: North America (Canada and the US), Latin America (Brazil, Chile, and Mexico), Europe (Czech Republic, Germany, the Netherlands, and Turkey), Asia (Japan) ranges from 3% in Japan to 16.9% in the USA (Andrade 2003). MDD has a chronic-intermittent course for about onethird (APA 2000) and psychotic features are present in 17% (Guze 1975). The clinical condition “psychotic depression (PD)” (Rothschild 2009), can indicate either a unipolar or bipolar depression accompanied by psychotic features. Prevalence of PD in the general population is estimated at 4 per 1,000 and highest among inpatients with a diagnosis of major depression (16-54%) (Rothschild 2009). The diagnostic focus of this review and condition under scrutiny will be unipolar depression i.e. MDD. That said, major mood disorders are claimed to form a spectrum from MDD to mania, and nearly 40% of those with MDD to have had a history of subthreshold hypomania (Angst 2010). Description of the intervention ECT is the application of an electric current to the head with the aim of inducing a controlled tonic-clonic convulsion or seizure, usually at daily intervals, to achieve an improvement in an abnormal mental state (Fink 1999). Treatment is often given in a series of eight to 12 sessions at a time. The induction of such cerebral seizure activity under anaesthetic conditions, known as modified ECT, is currently considered the standard ECT approach. ECT administered without anaesthesia, similar to the way the treatment was undertaken when it was first introduced in 1938 is known as unmodified ECT. Unmodified ECT is now considered unethical by many (Grunhaus 2010) and not in accordance with mainstream treatment guidelines (APA 2001; RCP 2005), although it is still practiced extensively today in many Asian countries (Chanpattana 2010) as well as in Russia and Africa (Ikeji 1999; Nelson 2005). International guidelines recommend that treatment should be provided in an ECT suite, where nursing, medical staff and a trained psychiatrist are present and the patient observed in a recovery room after treatment (RCP 2005). ECT devices emitting a brief-pulse wave electrical current are now recommended as the standard ECT treatment (RCP 2005). Brief-pulse devices have increasingly replaced the first sine-wave current machines (Abrams 2002; APA 2001; CPA 2001). Current dosage varies, depending on the electrode placement, either bitemporal, unilateral (right) or bifrontal (Kellner 2010). It has been claimed that cognitive side effects are reduced by using high dose right unilateral ECT, but autobio- Electroconvulsive therapy for depression (Protocol) Copyright © 2011 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd. 4 graphical memory loss is still reported as a problem (Fraser 2008). The use of ECT for depression is often a last resort when antidepressant medication is non-tolerable or in cases of medicationresistant relapsing severe major depression, and can be considered as life-saving in acute suicide-threatening and catatonic patients (Frederikse 2006). Where ECT is used as a first line of treatment, it is recommended that medication intolerance, previous positive response and medical status are taken into account (RCP 2005). How the intervention might work Although subject to much investigation, the mechanism of action for ECT is still not clearly established. Diverse neurochemical, neuroendocrine, electrophysiological and neurophysiological hypothesis have been proposed (CPA 2001; RCP 2005). Among these are the transient induction of increased pro-inflammatory cytokines (Lehtimaki 2008), increased expression of brain-derived neurotrophic factor (BDNF) (Taylor 2008), gene polymorphism and enhanced activity in the GABAergic, glutamate/glutaminergic and dopaminergic systems (Esel 2008; Huuhka 2008; Pfleiderer 2003). Influence on these systems are postulated to be linked to the effectiveness of ECT in Parkinson’s disease-related depression and in treatment-resistant depression (Huuhka 2008; Taylor 2008). In animal models, repeated electroconvulsive seizures have been found to enhance neurogenesis, synaptogenesis and remodelling of synapses in the hippocampus (Chen 2009) and recently the hippocampal volume in human subjects with depression has been found to increase after ECT (Nordanskog 2010). Taken together, ECT may have a positive effect on neuronal plasticity. Why it is important to do this review Despite the extensive use of ECT over the years, high quality evidence about the benefits and harms of ECT is still needed. Continued use of unmodified ECT in many countries may reflect erroneous clinical beliefs that cognitive deficits are transient and older treatment methods more efficient than modified ECT. A recent review about ECT knowledge, experience and attitudes includes studies on a very broad basis, regardless of study focus and design (Chakrabarti 2010). The 2002 Social Care Institute for Excellence (SCIE) report on consumers’ perspectives of ECT (Carr 2007) and the Lancet report from 2003 by the UK ECT Review group (UK ECT RG 2003) are both out of date. Scrutiny of sham-controlled ECT literature, assessing thoroughly the studies indicating a high placebo response rate (Rasmussen 2008) is also needed. Due to the high prevalence of depressive disorders, severity of symptoms, the relapsing condition, more updated knowledge on short- and long-term benefits, taking into account patient perspectives are needed. Differences between stimulus parameters (such as pulse amplitude, shape, width, frequency, duration) are impor- tant for understanding ECT outcomes (Peterchev 2010). Strong consumer perspectives (Rose 2003), concerns about use of continuation/maintenance ECT (Frederikse 2006) and discrepancy in reports of adverse effects (Feliu 2008; Porter 2008; Szabo 2007) all call for a comprehensive and systematic review to be undertaken. The use of quantitative and qualitative evidence together will maximize the use of primary data (Pearson 2004), offset the shortcomings of each type of evidence with the strengths of the other (Creswell 2004; Morse 2003), contribute to a comprehensive understanding and assist in the explanation of unexpected results (Johnson 2004). Nevertheless, discrepant qualitative and quantitative data will be treated with caution (Johnson 2004) and rather than placing greater value on one type of information the different types will be considered as complimentary (Moffatt 2006). The novel Integrative Methodological Approach, as described further in this review, will help to answer questions about the impact, appropriateness and acceptability of ECT and thereby enhance the scope, relevance and utility of the effectiveness review. The approach will not only expand the breadth of this review but enlighten the scientific discourse on mixed-methods review methodology. OBJECTIVES To assess the effects (benefits and harms) of ECT for depression in adults - and to integrate patient-lived experiences of ECT given for depression into the effectiveness review by novel methodology. (See sub-heading “The Integrative Methodological Approach” ). We will assess effects of ECT on a short- (immediately post treatment up to six months) and long-term (six months or more posttreatment commencement) basis, with special focus on long-term cognitive (memory) impairment. The review questions are: 1. Effectiveness 1.1 Effectiveness (benefits) How effective is ECT, on a short- and long-term basis, at relieving the symptoms of depression and at promoting full recovery of cognitive functioning (memory) and social functioning (activities of daily life) compared to other common treatments for depression (e.g. psychotherapy/cognitive behavioral therapy (CBT), antidepressants)? • Are there specific gender differences* and/or sub-groups of patients, such as voluntary versus involuntary*, treatment- Electroconvulsive therapy for depression (Protocol) Copyright © 2011 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd. 5 resistant or psychotic forms of depressive illness, who respond better to ECT compared to other treatments? • Are there differences in the efficacy of various forms of ECT (electrode placement, dose, waveform and frequency)? • Is the effect of ECT sustainable over time? • Is there evidence to support the use of continuation/ maintenance ECT? procedure, including during the administration of anaesthetic? 2. Patient experiences What is the lived experience of ECT and how do patients perceive the effect of ECT as a therapy? The Integrative Methodological Approach 1.2 Adverse effects (harm) How harmful is ECT, on both a short- and long-term basis, especially with regards to inducing transient or permanent cognitive impairment? • Are the adverse effects (i.e. neuropsychological impairment) of ECT different for the different forms (e.g. bilateral, unilateral or high-dose, low-dose) of ECT? • Are there differences in the rates of adverse events secondary to various forms of ECT, e.g. bilateral versus right-unilateral, high-dose v. low-dose? • What are the direct risks (i.e. harms*) of ECT (e.g. grand mal seizure), which could be experienced at the time of the actual We will conduct a systematic review which integrates qualitative evidence of the individual patient’s experience of the intervention (i.e. ECT as a treatment for depressive illness) into the effectiveness review. The approach was developed by the review team specifically for this systematic review question and was informed by Chapter 20 of the Cochrane Handbook of Systematic Reviews of Interventions (Noyes 2008). We will use qualitative data in parallel syntheses and juxtaposed alongside quantitative data, in order to i) inform and ii) extend the effectiveness review, as defined in Chapter 20 of the Handbook. The integrative four-phase methodological approach that we will undertake in this review is described in further detail in Appendix 1 and illustrated in Figure 1. Figure 1. The Integrative Methodological Approach Electroconvulsive therapy for depression (Protocol) Copyright © 2011 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd. 6 We will treat qualitative and quantitative evidence as two separate streams during the data extraction process: 1. Effectiveness (benefits and harm) and 2. Patient experiences. However, in phase four in order to aid the interpretation of synthesized quantitative evidence we will juxtapose the qualitative evidence alongside it. We will mark text added during phase two (refinement of the protocol) with an asterisk (*) in the revised protocol. Results from the qualitative evidence aiming to i) inform the effectiveness review by refining the protocol’s research questions and population, intervention, comparison and outcome (PICO) elements are given in Appendix 2. This is the revised version of the protocol, and we have undertaken the protocol refinement based on the qualitative evidence in two main areas: population (P) and outcome (O). Inclusion: we will include qualitative published studies of “first hand accounts”, without limit to designs such as phenomenology, grounded theory and ethnography. Exclusion: we will exclude second hand accounts, such as novels and historical literature. General inclusion and exclusion criteria of all studies We will include mixed methods studies that incorporate both quantitative and qualitative components where the research design matches the nominated study designs. We will not be blinded to the authors or other information about the publication when assessing the studies. If essential information about study quality or other information about a study is missing, we will contact the author(s) of the study by letter or email to obtain the information needed. METHODS Criteria for considering studies for this review Types of studies There will be no restrictions on publication date, geographic area or language on the bibliographic database search for eligible studies. Inclusion and exclusion criteria 1.1 Effectiveness (benefits) Inclusion: we will include only randomised controlled trials (RCTs) (single- or double-blind), including cross-over RCTs, cluster RCTs and quasi-RCTs; we will exclude all other study designs. Exclusion: we will exclude studies that are not RCTs, such as cohort studies, case-control, case series, pre- and post-intervention. We will make exception to this rule by including prospective long term follow-up studies (non-RCTs) on the following two topics: 1) cognitive functioning and 2) continuation/maintenance ECT. (We will treat included RCTs and non-RCTs separately; see Data collection and analysis.) 1.2 Adverse effects (harm) Inclusion: we will include RCTs, including cluster RCTs and observational studies (non-RCTs), including controlled or uncontrolled cohort studies, case-control, large case series, pre- and postintervention, prospective follow-up. We will discuss evidence from the RCTs and non-RCTs separately. Exclusion: we will not consider clinical case reports or reports of individual spontaneous occurrences of the adverse event (structural damage or cognitive impairment). 2. Patient experiences Types of participants The participants of included studies in the effectiveness review must have a clinically defined primary diagnosis of unipolar depression. We will define depression according to diagnostic criteria of the Diagnostic and Statistical Manual, Fourth edition (DSMIV) (APA 2000) and equivalent diagnosis of the International Classification of Diseases, Ninth or Tenth revision (ICD-9 or ICD-10) (WHO 1993) (see Table Appendix 3). We will also use equivalent diagnoses according to earlier DSM/ICD and versions for studies of earlier dates, as well as the DSM-IV primary care version (APA 1995). We will clearly note the diagnostic category. We will include ECT given on the background of a primary depressive episode, with or without psychotic symptoms. We will analyse patients meeting the diagnostic criteria for unipolar depression accompanied by psychotic features (hallucinations and delusions), clinically termed “psychotic depression” separately. Where diagnostic categories are clearly mixed we will only include the study if the unipolar depressed participants are analysed separately. We will exclude ECT administered mainly and specifically due to a manic episode, clear bipolar disorder or substance mood disorder. However, for participants in all included qualitative studies concerning lived experiences of the ECT intervention, we will consider mention of the main indication for ECT having been depression sufficient. Since we are interested in ECT recipients who were at least 15 years of age at time of treatment, we will explicitly exclude children and limit age to < 65 years in the 1. Effectiveness RCT arm of the review. In the 1. Effectiveness non-RCT long term cognitive functioning and non-RCT maintenance/continuation ECT, we will apply ECT having been given (for the first time or at study baseline) < 65 years and without concomitant conditions of dementia. Electroconvulsive therapy for depression (Protocol) Copyright © 2011 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd. 7 Types of interventions Our intervention of main interest is contemporary recommended ECT, meaning modified brief-pulse ECT (RCP 2005). However since both modified sine-wave ECT and even unmodified ECT is still in use in many countries, these intervention types will also be considered. ’Unmodified’ means ECT applied without anaesthesia. ’Modified’ means ECT applied with a) brief general anaesthesia and b) skeletal muscle relaxant drug (to modify convulsive motor activity) - with the aim of inducing generalized cerebral seizure activity of a type that is associated with a tonic-clonic or grand mal convulsion activity. ’Sine-wave’ refers to a constant electrical current stimulus, delivered in waves (one negative wave and one positive wave) around 50 to 60 waves per second (Hertz (Hz)). ’Brief-pulse’ refers to a constant electrical current stimulus, delivered by a series of brief pulses, usually around one millisecond (ms) in length. We will include standard brief-pulse ECT, ranging from 1 ms to 0.5 ms and we will also consider ultra brief-pulse ECT, which is 0.4 ms and below. Recommended electrical dose is referred to as stimulus dosing, varying from 25-50 (millicoulombs) (mC) up to 270-800 mC on ECT machines with a range of up to 1000 mC. The recommended stimulus dose is calculated according to age using the following formula: Stimulus dose mC = 5 x age (e.g. 250 mC dose for a 50 year old woman). A wide range of seizure thresholds has been observed among patients of differing age and gender. Another major concern is the large variation in the ECT intervention per se. All major ECT forms and comparisons between them will therefore be considered, i.e. current dosage (high, low), wave form (sine wave, brief pulse, ultra-brief pulse), electrode placement (bilateral, bifrontal, unilateral left or right, bilateral frontotemporal), as well as frequency (two or three times a week) and total number (nine or 12 sessions in one course of treatment). Comparisons to simulated (sham) ECT and to pharmacotherapeutic agents for depression will also be undertaken. Since active controls may cause a different set of adverse effects, the harms analysis will also be stratified according to type of comparator e.g. drug alone, sham, electrode placement. Types of comparisons for 1.1 Effectiveness (benefits) • ECT compared to control (simulated or “sham” ECT) • Comparisons of different forms of ECT as determined by waveform, dose, frequency of administration and electrode placement • ECT versus pharmacotherapy (e.g. antidepressants, St John’s Wort) • ECT versus psychological therapies (e.g. CBT, interpersonal therapy) • ECT plus pharmacotherapy versus psychological therapies plus pharmacotherapy ECT compared to control/simulated will be undertaken first and thereafter other comparisons in the above list. Similar comparisons will be applied to adverse events. Types of outcome measures The primary outcomes are depressive symptoms and cognitive functioning. Primary outcomes 1.1 Effectiveness (benefits) The main outcome will be: reduction in symptoms of depression. We will measure symptomatic change in depression by means of validated psychometric scales that assess mood and vegetative symptoms, comparing pre-treatment scores with scores at the end of the course of ECT. When possible we will analyze these as continuous data. When cut-off levels (e.g. Hospital Anxiety and Depression Scale (HADS) (Zigmond 1983), depression sub-scale > 8) are reported due to clinical utility, we will analyze the data as dichotomous. We will define treatment response conventionally as an at least 50% reduction of depressive symptoms. Examples of validated psychometric scales are Hamilton Rating Scale for Depression (Ham-D) (Hamilton 1960), Montgomery-Åsberg Depression Rating Scale (MADRS) (Montgomery 1979), Clinical Global Impression (CGI) (Guy 1976;Spearing 1997), Hospital Anxiety and Depression Scale (HADS) depression sub-scale (Zigmond 1983) and Beck Depression Inventory (BDI) (Beck 1961). We will regard observer-rated scales (e.g. Ham-D) separately from self-report scales (e.g. BDI). 1.2 Adverse effects (harm) The main outcome will be: reduction of cognitive (neuropsychological) functioning. • short-term (immediate, up to six months post treatment) neuropsychological consequences of ECT • long-term (six months or more post treatment) neuropsychological consequences of ECT Main outcome measures of interest are rate of cognitive recovery and change (impairment) in test scores relative to baseline (pre ECT) at time of discharge and follow-up. We will consider both continuous and binary outcome data. We will also take normbased comparisons of scores into account. Likewise, we will examine the adverse effects on cognitive (memory) functioning attributable to ECT and not to the depressive condition per se. We will measure cognitive functioning by means of validated neuropsychological tests. We will address neuropsychological performance and tests included according to the following domains: • Orientation (e.g. The Mini Mental State Examination (MMSE) (Folstein 1975)) Electroconvulsive therapy for depression (Protocol) Copyright © 2011 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd. 8 • Full scale IQ (e.g. Weschler Adult Intelligence Scale (WAISIII) (Wechsler 1955)) • Verbal and non-verbal memory function (Wechsler 1987), subdivided into a) anterograde (new learning) (for verbal e.g. The Buschke Selective Reminding Test (SRT) (Buschke 1973) and for non-verbal, e.g. Rey-Osterrieth complex figure reproduction (Rey 1941) b) retrograde (recall), including autobiographical memory (recall of personal events) (Kopelman 1989) 2. Patient experiences of ECT We will use qualitative research to identify the self-reported perspectives of patients receiving ECT, in terms of the impact of the event on emotional functioning and on both short- and long-term cognitive (memory) function after the end of treatment. The qualitative data of interest is that collected by interviews or focus groups, where these either are recorded and transcribed or extensive notes taken (not qualitative data analysed quantitatively), see Appendix 4. Secondary outcomes 1.1. Effectiveness (benefits) • disability (Sheehan Disability Scale (SDS)) (Sheehan 1983; Rush 2007) • psychosocial functioning (DSM-IV, Axis V, Global Assessment of Functioning Scale (GAF)) (APA 2000) • quality of life (Wisconsin Quality of Life Index (W-QLI)) (Becker 1993) 1.2 Adverse effects (harm) • rare events (mortality by any cause, cause-specific mortality, cerebral haemorrhage) • overall drop out as a proxy measure of overall acceptability of treatment • drop-out rate due to side effects • number of patients reporting at least one adverse event e.g. post ECT headache • distress (Impact of Event Scale (IES)) (Horowitz 1979) • anxiety (Hamilton Rating Scale for Anxiety (Ham-A)) (Hamilton 1959), Beck Anxiety Inventory (BAI) (Beck 1988), Clinical Anxiety Scale (CAS) (Snaith 1982) • subjective side effects (Columbia ECT Subjective Side Effects Schedule (CESSES)) (Sackeim 1987) • long-term structural brain damage or cellular degeneration, attributable to ECT and not to other underlying causes Search methods for identification of studies We will carry out electronic searches of bibliographic databases. There will be no publication, geographic, or language restrictions. Electronic searches Searches will cover the following sources: • Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library) • MEDLINE • EMBASE • CINAHL • PsycINFO • ISI Web of Knowledge • SveMed+ • OVID Nursing • British Nursing Index and Archive • Cochrane Collaboration Depression Anxiety and Neurosis group trials and reference databases (CCDAN) • Sigle Search terms We will undertake an extensive search for both qualitative and quantitative literature. The search terms listed in Appendix 5 are intended for MEDLINE, and we will adapt as required for other databases. Appendix 6 lists the terms that we will use for searching the CCDAN registers. Searching other resources Handsearches We will handsearch Convulsive Therapy (1985-1997) continued as Journal of ECT (1998 +) (ISSN 1095-0680). We will also handsearch the reference lists of the reports by the Task Force of the American Psychiatric Association (APA 2001) the Royal College of Psychiatrists’ Special Committee on ECT (RCP 2005) specialist textbooks such as Electroshock (Fink 1999), Electroconvulsive Therapy (Abrams 2002), Electroconvulsive and Neuromodulations Therapies (Swartz 2009) and Clinical Manual of Electroconvulsive Therapy (Mankad 2010). We will check the cited references of included studies and the systematic reviews identified by the DARE search for additional trials. Personal contacts We will make contact with experts in the field, independent researchers and manufacturers of ECT machines in order to identify unpublished reports, grey literature and ongoing studies. Electroconvulsive therapy for depression (Protocol) Copyright © 2011 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd. 9 Cross-referencing of bibliographies We will scan references in reviews and primary studies to identify new leads. the lead author of the publication by email in order to attempt to retrieve the necessary data for the analysis. Data extraction 1. Effectiveness (benefits and harm) Data collection and analysis The integrative methodological approach is described previously and in detail in Appendix 1, Appendix 2 and Figure 1. Paired reviewers (KAL, BH, LJS, GS, RB, KTH) will extract data (concerning study design, participant characteristics, intervention and outcomes) from the included studies. We will enter relevant information into a table of included studies. Data extraction 2. Patient experiences Selection of studies Paired reviewers (KAL, RB, SØ, GS, BH) will first check the titles independently and, where available, the abstracts of the studies identified by the electronic database searches. We will upload all references (both quantitative and qualitative) meeting the inclusion criteria for this first level of title and abstract screening into The DistillerSR online software programme (http://systematicreview.net/), a program specially developed for systematic review screening and data extraction by Evidence Partners Incorporated, 5584 Whitewood Ave., Manotick, ON K4M 1C9. DistillerSR allows for filtering of references along separate streams, screening according to specific inclusion and exclusion criteria, uploading of full text articles, data extraction according to specified forms and exporting of results (including an overview of excluded studies). After uploading into DistillerSR, the screening of references will proceed along two main separate streams, either 1. Effectiveness (benefits and harm) review (quantitative data) or 2. Patient experiences (qualitative data). We will further filter the 1. Effectiveness review stream into three arms: 1) RCTs and 2) non-RCTs about cognitive function (long-term harm (memory) impairment) 3) non-RCTs about maintenance/continuation ECT. We will thus keep results and evidence generated from all streams (qualitative and quantitative), as well as RCTs and non-RCTs separate, as recommended by the Handbook, Chapter 13 (Reeves 2008). However, we will integrate data about effectiveness and data about experiences in the last step. We will undertake screening of qualitative studies and qualitative data extraction (2. Patients’ experiences) first and thereafter protocol refinement, before proceeding on to the quantitative full text screening and data extraction (1. Effectiveness review). Data extraction and management We will request all references that appear to meet the inclusion criteria, and those where insufficient details exist to exclude. Pairs of two reviewers (RB, GS, KAL, BH, LJS) will undertake all abstract screening, full text inclusion/exclusion and data extraction in DistillerSR. We will resolve disagreements as to whether a study should be included by discussion with a third reviewer (KAL, BH, RB, GS, KTH). If we find that outcome data or other vital information are missing from the original reports, we will contact Two reviewers (RB, KAL, BH) will independently screen full text (Appendix 4), extract and summarize qualitative data, by means of an extraction form developed for this purpose (Appendix 7). We will include information about population, the type of ECT treatment received, and themes and conclusions of the research author(s) in the form. We will pool data, and if necessary (depending on the amount) we will use the Joanna Briggs Institute Qualitative Assessment and Review Instrument (JBI-QARI) (JBI 2007). In effect we will use a simplified content analysis approach to identify key concepts in the qualitative studies where patient experiences may help to enhance understanding of clinical outcomes. We will categorize the pooled and extracted units according to group similarity and frequencies of these determined categories. We will use dominant themes to form a list of suggestions for refinement of the protocol, on review questions and participants, interventions, comparators and outcomes (PICO) components. We will draft and discuss the list of refinement strategies until we reach consensus. We will incorporate the refinement strategies elements thereby agreed on into the protocol, and we will carefully detail the changes made. These changes may modify the study inclusion and exclusion criteria for the quantitative studies. Assessment of risk of bias in included studies 1. Effectiveness review Two reviewers (KAL, BH, LJS, GS, RB, KTH) will together conduct critical appraisal of the quantitative studies. We will assess all identified trials according to methodological aspects of trial design that have been shown to affect the validity of the results. The authors will not be blind to the authorship and source of the papers, as this procedure has been found to have no important impact on the overall assessment of study quality (Berlin 1997). We will not attempt to quantify study quality with a rating scale because the validity and reliability of such scales is uncertain (Juni 1999) but instead will describe: allocation concealment, blinding and number lost to follow-up. We will use Cochrane critical appraisal checklists appropriate for the type of study, i.e. RCT or observational (cohort study, case control etc.). Studies included for examining long term 1.2 Adverse effects (harm) must clearly state Electroconvulsive therapy for depression (Protocol) Copyright © 2011 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd. 10 how they identified the adverse event and cognitive impairment to be related to the ECT. We will resolve any disagreements as to whether a study should be included by discussion with a third reviewer (KAL, BH, LJS, GS, RB, KTH). 1.1 Effectiveness (benefits) We will assess risk of bias for each included RCT using The Cochrane Collaboration ’risk of bias’ tool (Higgins 2008a). We will consider the following six domains: 1. Sequence generation: was the allocation sequence adequately generated? 2. Allocation concealment: was allocation adequately concealed? 3. Blinding of participants, personnel and outcome assessors for each main outcome or class of outcomes: was knowledge of the allocated treatment adequately prevented during the study? 4. Incomplete outcome data for each main outcome or class of outcomes: were incomplete outcome data adequately addressed? 5. Selective outcome reporting: are reports of the study free of suggestion of selective outcome reporting? 6. Other sources of bias: was the study apparently free of other problems that could put it at a high risk of bias? Additional items to be included here are therapist qualifications, treatment fidelity and researcher allegiance/conflict of interest. A description of what was reported to have happened in each study will be provided, and a judgement on the risk of bias will be made for each domain within and across studies, based on the following three categories: low risk of bias, unclear risk or bias, high risk of bias. Two independent review authors will assess the risk of bias in selected studies (KAL, RB, GS, BH, LJS). We will discuss any disagreement with a third review author of the team (KAL, RB, GS, BH, LJS). Where necessary, we will contact the authors of the studies for further information. We will present all risk of bias data graphically and describe it in the text. We will use allocation concealment as a marker of trial quality for the purposes of undertaking sensitivity analyses. 1.2 Adverse effects (harm) We will assess all RCTs using the Risk of Bias tool described in the above section. For non-randomised studies (including prospective cohort studies, case-series, case-control studies, and pre-and post intervention design studies) we will use similar bias assessment principles, with a focus on selection bias, including the risk of bias due to confounding. This process is recommended by the Cochrane Handbook, Chapter 13 (Reeves 2008). We will consider and customize items used in the Newcastle-Ottawa Scale (NOS) for assessing non-randomized trials (Wells 2008) to the topic in question, either long-term continuation/maintenance ECT (benefit) or long-term cognitive impairment (harm). We will consider possible confounders such as age, gender, marital status, socioeconomic status, life events, other (co-morbid) physical illness (see Risk of bias table for non-RCT (cognitive function) - Appendix 8). 2. Patient experiences Two reviewers (RB, KAL, BH) will appraise the quality of the included qualitative studies by use of the Qualitative Assessment and Review Instrument developed by the Joanna Briggs Institute (JBI) (JBI 2007). We will not exclude studies on quality grounds, but we will use the outcome of the appraisal to give more weight to studies that achieve high quality rating and less weight to studies that achieve low quality rating. Measures of treatment effect 1. Effectiveness A single primary outcome for the assessment of the efficacy of ECT has been defined, a priori, in order to avoid the risk of multiple testing or data driven analyses (Freemantle 2001).This decision is based on methodological grounds and should not be taken to mean that the secondary outcomes are inherently of less interest. We will calculate overall effect sizes. We will compare the treatment and control groups for outcomes at post-test and at two different follow-up times (short- and long-term), when different time points are reported. For continuous data, we will use either mean differences (MD) or (when different scales are used) standardized mean differences (SMD) for absolute measures. For dichotomous data (binary outcome measures), we will use relative effect measures (odds ratio (OR), risk ratio (RR)) or absolute effect measures (risk difference (RD) or numbers needed to treat (NNT)). Since none of these measures are uniformly the best choice for dichotomous data, the decision will take into account consistency, mathematical properties and ease of interpretation. We will prefer relative effect measures (RR or OR) for undertaking meta-analyses and the results sought re-expressed using absolute effect measures (RD or NNT) in order to ease interpretation and clinical utility (Deeks 2008). Where necessary (if data on the same outcome are presented as dichotomous in some studies and continuous in others, as often can be the case for scores on depression scales) we will combine dichotomous and continuous data by statistical approaches (re-expressing ORs as SMD or visa versa) as described in the Handbook (Deeks 2008). We will report 95% confidence intervals for RD, OR and RR. Dealing with dependent outcomes In some primary studies, several different outcomes are measured on the same participants. Further, the same outcome may be measured at multiple points in time. Given the discrepancy between short- and long-term outcomes, we will opt for the longest possible Electroconvulsive therapy for depression (Protocol) Copyright © 2011 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd. 11 follow-up time, by using the most recent outcome of ECT treatment from each sample. Data will consist of one measure from a single point in time for each sample. Unit of analysis issues 1. Effectiveness We will consider the level at which randomisation occurred. For cluster-randomised trials, where groups of participants are randomised, we will take care to avoid unit of analysis errors. If we include any cluster RCTs in this review, we will attempt to measure the intra-cluster correlation (ICC). The total variance in the outcome can be partitioned into variance between groups (VBG) and variance within groups (VWG). The ICC is calculated as VBG/ (VBG+VWG). But the ICC is seldom reported in the primary studies. The number of participants can be used in the analyses if the ICC is used as a correcting factor. For dichotomous data both the number of participants and the number experiencing the event can be divided by the same design effect (Higgins 2002). This review may lead to issues with timing of outcome measurement that could be considered unit of analysis issues. The protocol defines the time periods for assessment of outcomes as being either short- or long-term, with short-term being less than six months, while long-term is greater than six months. For repeated observations over time (studies of long duration) the long-term data of choice will be at 12 or 24 months post intervention. We will not duplicate studies across multiple time frames. In cases with several treatment arms, we will compare only one of the treatment arms with the control group. Dealing with missing data 1. Effectiveness We will base missing data according to drop-out on intention to treat data (ITT) when this is obtainable; that is, ’the principle that asserts that the effect of a policy can be best assessed by evaluating on the basis of the intention to treat a subject (i.e. the planned treatment regimen) rather than the actual treatment received’ ( ICH 1999) - otherwise we will use completers’ analysis (where subjects included have actually received the course of treatment that they were allocated to). We will deal with missing data along four lines (Higgins 2008b): 1) authors contacted to request missing data; 2) assumptions made of the methods used to cope with missing data, such as categorizing into ‘missing at random’ and ‘not missing at random.’ Data fall into the ‘missing at random’ category when they are missing unrelated to the actual values of the missing data (i.e. if the respondent forgets by chance to answer a question on the questionnaire, it will fall into this category). Data fall into the ‘not missing at random’ category when the missing is related to the actual missing data (i.e. if the respondent intentionally omits or does not answer a question due to some reason, e.g. the question being too sensitive, it will fall into this category). In cases where we assume that data are missing at random, we will analyze the available data only. If we assume that the data are not missing at random, we will impute the missing data with replacement values, and treat these as if they were observed. We will do this in different ways and compare the results (e.g. last observation carried forward, imputing an assumed outcome such as assuming all were poor outcomes, imputing the mean, imputing based on predicted values from a regression analysis) 3) by the assumptions made, assess how sensitive results are to reasonable changes (sensitivity analyses) 4) evaluate the potential impact of missing data on the findings of the review. Assessment of heterogeneity 1. Effectiveness When there is statistically significant heterogeneity among primary outcome studies, the following factors are considered as possible explanations: sine-wave ECT, brief or ultra-brief pulse type of ECT, unilateral or bilateral, and differences in dosage and in participant characteristics. If the primary studies are too heterogeneous to be grouped according to these characteristics, we will not perform a meta-analysis. If there are many primary studies, we will classify them according to these variables in order to identify possible sources of heterogeneity. We will consider performing moderator analyses (stratification on subgroups, meta-analysis analogue to ANOVA, meta-regression) to explore how observed variables are related to heterogeneity. We will assess statistically significant heterogeneity among primary outcome studies with Chi2 (Q) test and I2 . We will quantify inconsistency using the formula I2 = ((Q - df )/Q) x 100% where Q is Chi2 and df is degrees of freedom (Higgins 2002; Higgins 2003). < We will consider a significant Q (P = .05) and I2 of at least 50% as statistical heterogeneity (Deeks 2008). Assessment of reporting biases 1. Effectiveness We will use funnel plots to explore the likelihood of publication bias. Asymmetry of the funnel plot may indicate possible publication bias in this review, but may also indicate other methodological or sample size issues within the trials. If we find asymmetry of the funnel plot, we will examine the clinical diversity of the studies (Egger 1997). Electroconvulsive therapy for depression (Protocol) Copyright © 2011 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd. 12 Data synthesis 1. Effectiveness We will enter relevant information into a table of included studies. We will conduct meta-analyses when warranted. If we judge metaanalyses unsuitable, we will report results for each individual study narratively. We will use both fixed-effect and random-effects metaanalyses. For continuous data we will undertake the inverse-variance fixedeffect or inverse-variance random-effect meta-analyses method. We will consider the possibility of skewed data and heterogeneity. If there are sufficiently homogenous studies we will prefer the fixedeffect MD or SMD method (Deeks 2008). When in doubt we will use the more conservative random-effects method (giving a wider confidence interval (CI) and less significant P value) approach. We will take care to use the appropriate means and standard deviations (of either change from baseline or final measurements). For dichotomous data, we will utilize three fixed-effect methods (Mantel-Haenszel, Peto or Inverse variance) or one random-effects method (DerSimonian and Laird). We will present the method used clearly in the text of the review. If studies are sufficiently homogenous, we will prefer the Mantel-Haenszel method, since this method is more robust when study data are sparse (events few or study size small). We will consider the Peto method (for pooling of OR) when events are very rare or for the purpose of combining studies using time-to-event analyses and log rank tests (Deeks 2008). If there is statistically significant heterogeneity among studies’ effect sizes, we will use the random-effects method (DerSimonian and Laird). We will test homogeneity with the Q-test (Chi2 , P value) and measure the degree of heterogeneity with I2 (Higgins 2003). However, the decision will not rest entirely on the outcome of these procedures. We will also take clinical diversity and the nature of the measures into account, as well as the aim in question, as for providing a measure of the average intervention effect (random-effects estimate) or the best intervention effect (fixed-effect estimate). We will also keep the amount of between-study variation (measured by the Mantel-Haenszel method) as opposed to the amount of variation across studies (measured by DerSimonian and Laird method) in mind. 2. Patient experiences We will pool the qualitative research findings separately from the quantitative effectiveness findings as described in the integrative approach, phase one and three (Appendix 1; Figure 1). This will involve an aggregation of findings to generate a set of statements that represent that aggregation, through assembling the findings based on similarity of meaning or intent. This is essentially an interpretive process. We will specifically examine the existence of a set of themes decided in advance of analysing the data (we outline the effectiveness questions and a list of outcome variables of interest prior to qualitative data analysis). That is, we will work “down” from pre-existing understanding (Ezzy 2002) and seek out evidence from qualitative studies to address questions directly related to the effectiveness review. Thus, we will seek themes from each qualitative study that directly relate to the outcomes of interest. The aggregation of findings leads to the development of categories. These categories are then aggregated in order to produce a single synthesis of findings that provides a new perspective on perceived benefits and harms of ECT and a better understanding of outcomes from the patients’ perspective. For each step in the analysis, we will select illustrative quotations (two or three) from the qualitative studies for each category. Where textual pooling is not possible we will present the findings in narrative form. In phase four of the integrative approach, we will report the quantitative results as is standard, and present qualitative data alongside this. We will report the results of the descriptive qualitative synthesis, highlighting higher order thematic descriptions. We will present illustrative quotations (two to three) from the qualitative studies for each higher order description. We will choose the quotations to represent views that appear frequently, thus describing the patients’ opinions on and experiences with these particular situations. They will give an impression of not just what the users’ opinion is about ECT but an understanding of why they feel the way they do (Popay 2005). This method of integrating the qualitative categories with already established quantitative outcomes rests within the aim of extending the effectiveness review. Subgroup analysis and investigation of heterogeneity In order to examine if the ECT intervention effect varies in different populations (e.g. such as males and females) or by intervention characteristics (such as current dose, type of ECT or electrode placement), we will undertake subgroup analyses and metaregression when feasible. We will take particular interest in the analysis of clinical heterogeneity, by sub-grouping the studies of unipolar depression: • men or women • with or without psychotic features (i.e. hallucinations, delusions) • with or without catatonic features (i.e. stupor, motoric immobility) • with or without postpartum onset • with or without chronicity (i.e. long term illness, 2 years or more, without inter-episode full remission) • with or without medication-resistant depression • with or without forced (involuntary) treatment or involuntary hospital admission* We will undertake investigation of heterogeneity as earlier described (under heading Assessment of heterogeneity). We will set the number of studies considered sufficient for simple regression analyses higher (at least 10) when co-variates are unevenly distributed. We will also keep the number of characteristics investigated small in order to reduce the likelihood of a false positive Electroconvulsive therapy for depression (Protocol) Copyright © 2011 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd. 13 result. We will consult a statistical expert familiar with the many pitfalls of subgroup analyses (Deeks 2008) for all subgroup analyses undertaken and also when in doubt. diagnostic comorbidity (i.e. MDD and 30-40% or more comorbidity with e.g. anxiety) Sensitivity analysis ACKNOWLEDGEMENTS If the number of included studies is sufficient (more than 10), we will assess the impact of differing methodological quality by sensitivity analyses. The following sensitivity analyses are planned a priori. By limiting the studies to be included to those with higher quality, we will examine if the results change, and check for the robustness of the observed findings. 1. Excluding quasi randomised studies from the analysis. 2. Excluding where the drop out rate is greater than 20%. 3. Performing the worst case scenario ITT (all the participants in the experimental group experience the negative outcome and all those allocated to the comparison group experience the positive outcome) and the best case scenario ITT (all the participants in the experimental group experience the positive outcome and all those allocated to the comparison group experience the negative outcome). 4. Removing studies that include patients with psychotic and/ or catatonic features. 5. Removing studies that include patients where the majority (over 80%) but not all meet the specified age-range (between 15 and 65 years). 6. Removing studies that include patients with a high degree of Many thanks to Janet Harris (Section of Public Health, School of Health and Related Research, University of Sheffield, UK) who, since the protocol idea was conceived, has given her advice on the initial reading of qualitative studies in order to enhance the objectives and research questions of the effectiveness review. We also extend our thanks to Alan Pearson and Craig Lockwood (Joanna Briggs Institute, Adelaide, Australia) who have participated in early protocol idea discussions and generously given their advice on the synthesis of qualitative evidence within the quantitative systematic review. Thanks to Kjell Martin Moksnes (Senior consultant, Head of department, Ullevål University Hospital, Psychogeriatric Department Vardåsen, Oslo, Norway) who has generously provided his in depth expert clinical experience and concerns about best clinical practice and the need for updated evidence about ECT’s benefits and adverse effects. Thanks to John Geddes, MD, FRCPsych (Centre for EvidenceBased Mental Health, Department of Psychiatry, University of Oxford) who has given permission to build on the previous withdrawn Cochrane protocol draft. REFERENCES Additional references Abrams 2002 Abrams R. Does brief-pulse ECT cause persistent or permanent memory impairment?. The Journal of ECT 2002;18(2):71–3. Andrade 2003 Andrade L, Caraveo-Anduaga JJ, Berglund P, Bijl RV, De Graaf R, Vollebergh W, et al.The epidemiology of major depressive episodes: results from the International Consortium of Psychiatric Epidemiology (ICPE) Surveys. International Journal of Methods in Psychiatric Research 2003;12(1):3–21. Angst 2010 Angst J, Cui L, Swendsen J, Rothen S, Cravchik A, Kessler RC, et al.Major depressive disorder with subthreshold bipolarity in the National Comorbidity Survey Replication. The American Journal of Psychiatry 2010;167(10):1164–201. APA 1995 American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Primary Care Version (DSM-IV-PC). 4th Edition. Washington, DC: American Psychiatric Association, 1995. APA 2000 American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Text Revision (DSM-IV-TR). 4th Edition. Washington, DC: American Psychiatric Association, 2000. APA 2001 American Psychiatric Association. In: Weiner Richard D editor(s). The Practice of Electroconvulsive Therapy: Recommendations for Treatment, Training, and Privileging: A Task Force Report of the American Psychiatric Association. 2nd Edition. Washington, DC: American Psychiatric Association, 2001. Beck 1961 Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Archives of General Psychiatry 1961;4:561–71. Beck 1988 Beck Aaron T, Epstein Norman, Brown Gary, Steer Robert A. An inventory for measuring clinical anxiety: Psychometric properties. Journal of Consulting and Clinical Psychology 1988;56(6):893–7. Becker 1993 Becker M, Diamond R, Sainfort F. A new patient focused index for measuring quality of life in persons with severe and persistent Electroconvulsive therapy for depression (Protocol) Copyright © 2011 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd. 14 mental illness. Quality of Life Research 1993;2(4):239–51. Berlin 1997 Berlin JA. Does blinding of readers affect the results of metaanalyses? University of Pennsylvania Meta-analysis Blinding Study Group. Lancet 1997;350(9072):185–6. Buschke 1973 Buschke H. Selective reminding for analysis of memory and learning. Journal of Verbal Learning and Verbal Behavior 1973;12 (5):543–50. Carr 2007 Carr S, Fleischmann P. Systematic review of consumers’ perspectives on electro-convulsive therapy. In: Carr S, Coren E editor(s). Collection of examples of service user and carer participation in systematic reviews. London: Social Care Institute for Excellence, 2007:4–13. Chakrabarti 2010 Chakrabarti S, Grover S, Rajagopal R. Electroconvulsive therapy: a review of knowledge, experience and attitudes of patients concerning the treatment. World Journal of Biological Psychiatry 2010;11(3):162–74. Chanpattana 2005 Chanpattana W, Kojima K, Kramer BA, Intakorn A, Sasaki S, Kitphati R. ECT practice in Japan. The Journal of ECT 2005;21 (3):139–44. Chanpattana 2007 Chanpattana W. A questionnaire survey of ECT practice in Australia. The Journal of ECT 2007;23(2):89–92. Chanpattana 2010 Chanpattana W, Kramer BA, Kunigiri G, Gangadhar BNM, Kitphati R, Andrade C. A Survey of the Practice of Electroconvulsive Therapy in Asia. The Journal of ECT 2010;26(1): 5–10. Chen 2009 Chen F, Madsen TM, Wegener G, Nyengaard JR. Repeated electroconvulsive seizures increase the total number of synapses in adult male rat hippocampus. European Neuropsychopharmacology 2009;19(5):329–38. CPA 2001 Enns MW, Reiss JP. Canadian Psychiatric Association ECT Position Paper, 2001. http://ww1.cpa-apc.org:8080/publications/ position˙papers/Therapy.asp (accessed 21 September 2010). CRD 2009 Centre for Reviews and Dissemination. Systematic reviews: CRD’s guidance for undertaking reviews in health care. http:// www.york.ac.uk/inst/crd/pdf/Systematic˙Reviews.pdf. York: CRD, University of York, (accessed 21 September 2010). Creswell 2004 Creswell JW, Fetters MD, Ivankova NV. Designing a mixed methods study in primary care. Annals of Family Medicine 2004;2 (1):7–12. Deeks 2008 Deeks JJ, Higgins JPT, ALtman DG (editors). Chapter 9: Analysing data and undertaking meta-analyses. Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.1 [updated September 2009]. The Cochrane Collaboration, 2008. Available from www.cochranehandbook.org. Egger 1997 Egger M, Davey Smith G, Schneider M, Minder C. Bias in metaanalysis detected by a simple, graphical test. BMJ 1997;315(7109): 629–34. Eranti 2003 Eranti SV, McLoughlin DM. Electroconvulsive therapy - state of the art. British Journal of Psychiatry 2003;182:8–9. Esel 2008 Esel E, Kose K, Hacimusalar Y, Ozsoy S, Kula M, Candan Z, et al.The effects of electroconvulsive therapy on GABAergic function in major depressive patients. The Journal of ECT 2008;24(3): 224–8. Ezzy 2002 Ezzy D. Qualitative analysis: practice and innovation. London: Routledge, 2002. Feliu 2008 Feliu M, Edwards CL, Sudhakar S, McDougald C, Raynor R, Johnson S, et al.Neuropsychological effects and attitudes in patients following electroconvulsive therapy. Neuropsychiatric Disease and Treatment 2008;4(3):613–7. Fink 1999 Fink M. Electroshock: Restoring the Mind. New York, NY: Oxford University Press, 1999. Folstein 1975 Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research 1975;12(3):189–98. Fraser 2008 Fraser LM, O’Carroll RE, Ebmeier KP. The effect of electroconvulsive therapy on autobiographical memory: a systematic review. The Journal of ECT 2008;24(1):10–7. Frederikse 2006 Frederikse M, Petrides G, Kellner C. Continuation and maintenance electroconvulsive therapy for the treatment of depressive illness: a response to the National Institute for Clinical Excellence report. The Journal of ECT 2006;22(1):13–7. Freemantle 2001 Freemantle N. Interpreting the results of secondary end points and subgroup analyses in clinical trials: should we lock the crazy aunt in the attic?. BMJ 2001;322(7292):989–91. Gazdag 2009 Gazdag G, Palinska D, Kloszewska I, Sobow T. Electroconvulsive therapy practice in Poland. The Journal of ECT 2009;25(1):34–8. Grunhaus 2010 Grunhaus L. A call for action - my own personal views. The Journal of ECT 2010;26(1):32–3. Guy 1976 Guy W. ECDEU Assessment manual for psychopharmacologyrevised. [DHHS Publ No ADM 91-338]. Rockville, MD, U.S.: Department of Health and Human Services, 1976:218–22. Electroconvulsive therapy for depression (Protocol) Copyright © 2011 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd. 15 Guze 1975 Guze SB, Woodruff RA Jr, Clayton PJ. The significance of psychotic affective disorders. Archives of General Psychiatry 1975;32 (9):1147–50. Hamilton 1959 Hamilton M. The assessment of anxiety states by rating. British Journal of Medical Psychology 1959;32(1):50–5. Hamilton 1960 Hamilton M. A rating scale for depression. Journal of Neurology, Neurosurgery and Psychiatry 1960;23:56–62. Higgins 2002 Higgins JP, Thompson SG. Quantifying heterogeneity in a metaanalysis. Statistics in Medicine 2002;21(11):1539–58. Higgins 2003 Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327(7414):557–60. Higgins 2008a Higgins JPT, Altman DG (editors). Chapter 8: Assessing risk of bias in included studies. Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.1 [updated September 2009]. The Cochrane Collaboration, 2008. Available from www.cochrane-handbook.org. Higgins 2008b Higgins JPT, Deeks JJ, Altman DG (editors). Chapter 16: Special topics in statistics. Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.1 [updated September 2009]. The Cochrane Collaboration, 2008. Available from www.cochrane-handbook.org. Horowitz 1979 Horowitz M, Wilner N, Alvarez W. Impact of Event Scale: a measure of subjective stress. Psychosomatic Medicine 1979;41(3): 209–18. Huuhka 2008 Huuhka K, Anttila S, Huuhka M, Hietala J, Huhtala H, Mononen N, et al.Dopamine 2 receptor C957T and catechol-omethyltransferase Val158Met polymorphisms are associated with treatment response in electroconvulsive therapy. Neuroscience Letters 2008;448(1):79–83. ICH 1999 ICH E9 Expert Working Group. International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use: ICH Harmonised Tripartite Guideline: Statistical principles for clinical trials. Statistics in Medicine 1999;18(15):1905–42. Ikeji 1999 Ikeji OC, Ohaeri JU, Osahon RO, Agidee RO. Naturalistic comparative study of outcome and cognitive effects of unmodified electro-convulsive therapy in schizophrenia, mania and severe depression in Nigeria. East African Medical Journal 1999;76(11): 644–50. Ingram 2008 Ingram A, Saling MM, Schweitzer I. Cognitive side effects of brief pulse electroconvulsive therapy: a review. The Journal of ECT 2008; 24(1):3–9. JBI 2007 The Joanna Briggs Institute. QARI, The Joanna Briggs Institute qualitative assessment and review instrument. Users Manual for version 4.0. SUMARI, The Joanna Briggs Institute system for the unified management, assessment and review of information. Version 4.0. Adelaide: The Joanna Briggs Institute, 2007:39–68. Johnson 2004 Johnson RB, Onwuegbuzie AJ. Mixed methods research: a research paradigm whose time has come. Educational Researcher 2004;33(7): 14–26. Johnstone 1999 Johnstone L. Adverse psychological effects of ECT. Journal of Mental Health 1999;8(1):69–85. Juni 1999 Juni P, Witschi A, Bloch R, Egger M. The hazards of scoring the quality of clinical trials for meta-analysis. JAMA 1999;282(11): 1054–60. Kellner 2010 Kellner CH, Tobias KG, Wiegand J. Electrode placement in electroconvulsive therapy (ECT): a review of the literature. The Journal of ECT 2010;26(3):175–80. Koopowitz 2003 Koopowitz LF, Chur-Hansen A, Reid S, Blashki M. The subjective experience of patients who received electroconvulsive therapy. Australian and New Zealand Journal of Psychiatry 2003;37(1):49–54. Kopelman 1989 Kopelman MD, Wilson BA, Baddeley AD. The autobiographical memory interview: a new assessment of autobiographical and personal semantic memory in amnesic patients. Journal of Clinical and Experimental Neuropsychology 1989;11(5):724–44. Lehtimaki 2008 Lehtimaki K, Keranen T, Huuhka M, Palmio J, Hurme M, Leinonen E, et al.Increase in plasma proinflammatory cytokines after electroconvulsive therapy in patients with depressive disorder. The Journal of ECT 2008;24(1):88–91. Little 2003 Little JD. ECT in the Asia Pacific region: what do we know?. The Journal of ECT 2003;19(2):93–7. Malekian 2009 Malekian A, Amini Z, Maracy MR, Barekatain M. Knowledge of attitude toward experience and satisfaction with electroconvulsive therapy in a sample of Iranian patients. The Journal of ECT 2009; 25(2):106–12. Mankad 2010 Mankad MV, Beyer JL, Weiner RD, Krystal A. Clinical Manual of Electroconvulsive Therapy. Washington, DC: American Psychiatric Publishing, Inc, 2010. McCall 2001 McCall WV. Electroconvulsive therapy in the era of modern psychopharmacology. International Journal of Neuropharmacology 2001;4(3):315–24. Moffatt 2006 Moffatt S, White M, Mackintosh J, Howel D. Using quantitative and qualitative data in health services research - what happens when Electroconvulsive therapy for depression (Protocol) Copyright © 2011 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd. 16 mixed method findings conflict?. BMC Health Services Research 2006;6:28. [: ISRCTN61522618] Montgomery 1979 Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. British Journal of Psychiatry 1979;134:382–9. Morse 2003 Morse J. Principles of mixed- and multi-method research design. In: Tashakkori A, Teddlie C editor(s). Handbook of Mixed Methods in Social & Behavioral Research. Thousand Oaks, CA: SAGE, 2003: 768. Nelson 2005 Nelson AI. A national survey of electroconvulsive therapy use in the Russian Federation. The Journal of ECT 2005;21(3):151–7. NICE 2003 NICE. Technology Appraisal Guidance 59: Guidance on the use of electroconvulsive therapy [April 2003]. Available from: http:// www.nice.org.uk/nicemedia/pdf/59ectfullguidance.pdf (accessed 11 April 2008). London: National Institute for Health and Clinical Excellence. Nordanskog 2010 Nordanskog P, Dahlstrand U, Larsson MR, Larsson E-M, Knutsson L, Johanson A. Increase in hippocampal volume after electroconvulsive therapy in patients with depression: A volumetric magnetic resonance imaging study. The Journal of ECT 2010;26(1): 62–7. Noyes 2008 Noyes J, Popay J, Pearson A, Hannes K, Booth A. Chapter 20: Qualitative research and Cochrane reviews. Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.1 [updated September 2009]. The Cochrane Collaboration, 2008. Available from www.cochranehandbook.org. Okagbue 2008 Okagbue N, McIntosh A, Gardner M, Scott AI. The rate of usage of electroconvulsive therapy in the city of Edinburgh, 1993-2005. The Journal of ECT 2008;24(3):229–31. Orr 2005 Orr A, O’Connor D. Dimensions of power: older women’s experiences with electroconvulsive therapy (ECT). Journal of Women & Aging 2005;17(1-2):19–36. Pearson 2004 Pearson A. Balancing the evidence: incorporating the synthesis of qualitative data into systematic reviews. JBI Reports 2004;2:45–64. Peterchev 2010 Peterchev AV, Rosa MA, Deng ZD, Prudic J, Lisanby SH. Electroconvulsive therapy stimulus parameters: rethinking dosage. The Journal of ECT 2010;26(3):159–74. Pfleiderer 2003 Pfleiderer B, Michael N, Erfurth A, Ohrmann P, Hohmann U, Wolgast M, et al.Effective electroconvulsive therapy reverses glutamate/glutamine deficit in the left anterior cingulum of unipolar depressed patients. Psychiatry Research 2003;122(3): 185–92. Popay 2005 Popay J. Moving beyond floccinaucinihilipilification: enhancing the utility of systematic reviews. Journal of Clinical Epidemiology 2005;58(11):1079–80. Porter 2008 Porter R, Heenan H, Reeves J. Early effects of electroconvulsive therapy on cognitive function. The Journal of ECT 2008;24(1): 35–9. Prudic 2001 Prudic J, Olfson M, Sackeim HA. Electro-convulsive therapy practices in the community. Psychological Medicine 2001;31(5): 929–34. Rajkumar 2007 Rajkumar AP, Saravanan B, Jacob KS. Voices of people who have received ECT. Indian Journal of Medical Ethics 2007;4(4):157–64. Rasmussen 2008 Rasmussen KG. Sham electroconvulsive therapy studies in depressive illness: a review of the literature and consideration of the placebo phenomenon in electroconvulsive therapy practice. The Journal of ECT 2008;25(1):54–9. RCP 2005 Royal College of Psychiatrists. The ECT Handbook: The Third Report of the Royal College of Psychiatrists’ Special Committee on ECT. London: Royal College of Psychiatrists, 2005. Reeves 2008 Reeves BC, Deeks JJ, Higgins JPT, Wells GA. Chapter 13: Including non-randomized studies. Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.1 [updated September 2009]. The Cochrane Collaboration, 2008. Available from www.cochrane-handbook.org. Rey 1941 Rey A. The psychological examination in cases of traumatic encepholopathy. Problems. Archives de Psychologie. Editions Medecine et Hygiene, 1941; Vol. 28:215–85. Rose 2003 Rose D, Fleischmann P, Wykes T, Leese M, Bindman J. Patients’ perspectives on electroconvulsive therapy: systematic review. BMJ 2003;326(7403):1363. Rothschild 2009 Rothschild AJ. Clinical Manual for Diagnosis and Treatment of Psychotic Depression. Washington, DC: American Psychiatric Publishing, Inc, 2009. Rush 2007 Rush AJ, First MB, Blacker D. Handbook of Psychiatric Measures. 2nd Edition. Washington, DC: American Psychiatric Publishing, Inc, 2007. Sackeim 1987 Sackeim HA, Ross FR, Hopkins N, Calev L, Devanand DP. Subjective side effects acutely following ECT: associations with treatment modality and clinical response. Convulsive Therapy 1987; 3(2):100–10. Sackeim 2007 Sackeim HA, Prudic J, Fuller R, Keilp J, Lavori PW, Olfson M. The cognitive effects of electroconvulsive therapy in community settings. Neuropsychopharmacology 2007;32(1):244–54. Electroconvulsive therapy for depression (Protocol) Copyright © 2011 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd. 17 Sheehan 1983 Sheehan DV. The Anxiety Disease. New York: Scribners, 1983:206. Sienaert 2005 Sienaert P, Filip B, Willy M, Joseph P. Electroconvulsive therapy in Belgium: a questionnaire study on the practice of electroconvulsive therapy in Flanders and the Brussels Capital region. The Journal of ECT 2005;21(1):3–6. Smith 2009 Smith M, Vogler J, Zarrouf F, Sheaves C, Jesse J. Electroconvulsive therapy: the struggles in the decision-making process and the aftermath of treatment. Issues in Mental Health Nursing 2009;30 (9):554–9. Snaith 1982 Snaith RP, Baugh SJ, Clayden AD, Husain A, Sipple MA. The Clinical Anxiety Scale: an instrument derived from the Hamilton Anxiety Scale. British Journal of Psychiatry 1982;141:518–23. Spearing 1997 Spearing MK, Post RM, Leverich GS, Brandt D, Nolen W. Modification of the Clinical Global Impressions (CGI) Scale for use in bipolar illness (BP): the CGI-BP. Psychiatry Research 1997;73: 159–71. Stek 2003 Stek M, Van der Wurff FB, Hoogendijk W, Beekman A. Electroconvulsive therapy for the depressed elderly. Cochrane Database of Systematic Reviews 2003, Issue 2. [DOI: 10.1002/ 14651858.CD003593] Sterling 2000 Sterling P. ECT damage is easy to find if you look for it. Nature 2000;403(6767):242. Swartz 2009 Swartz CM. Electroconvulsive and Neuromodulation Therapies. New York: Cambridge University Press, 2009. Szabo 2007 Szabo K, Hirsch JG, Krause M, Ende G, Henn FA, Sartorius A, et al.Diffusion weighted MRI in the early phase after electroconvulsive therapy. Neurological Research 2007;29(3):256–9. Taylor 2008 Taylor SM. Electroconvulsive therapy, brain-derived neurotrophic factor, and possible neurorestorative benefit of the clinical application of electroconvulsive therapy. The Journal of ECT 2008; 24(2):160–5. UK ECT RG 2003 The UK ECT Review Group. Efficacy and safety of electroconvulsive therapy in depressive disorders: a systematic review and meta-analysis. Lancet 2003;361(9360):799–808. Wechsler 1955 Wechsler D. Wechsler Adult Intelligence Scale Manual. New York: Psychological Corporation, 1955. Wechsler 1987 Wechsler D. Wechsler Memory Scale-Revised Manual. New York: Psychological Corporation, 1987. Wells 2008 Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, et al.The Newcastle-Ottawa Scale (NOS) for assessing the quality of non randomised studies in meta-analyses. www.ohri.ca/programs/ clinical˙epidemiology/oxford.htm (Accessed 20 February 2009). WHO 1993 World Health Organization. The ICD-10 Classification of Mental and Behavioural Disorders. Diagnostic Criteria for Research. Geneva: WHO, 1993. WHO 2008 World Health Organization. The global burden of disease: 2004 update. The Global Burden of Disease: 2004 Update. Geneva: WHO, 2008. Zigmond 1983 Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatrica Scandinavica 1983;67(6):361–70. ∗ Indicates the major publication for the study Electroconvulsive therapy for depression (Protocol) Copyright © 2011 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd. 18 APPENDICES Appendix 1. The Integrative Methodological Approach The effectiveness review will synthesize qualitative evidence in order to complement the review. Qualitative data will complement the effectiveness review for two purposes: • To inform the effectiveness review in the formulation and refining the research questions and population, intervention, comparison, and outcome (PICO) elements. • To extend the effectiveness review by integrating qualitative evidence that addresses questions directly related to the effectiveness review. The aims are consistent with the Cochrane Qualitative Research Methods Group (QRMG) recommendations in Chapter 20 of the Cochrane Handbook of Systematic Reviews of Interventions, Version 5.0.1 (Higgins 2008a) and Chapter 6 of the Centre for Review and Dissemination’s (CRD) guidance for undertaking reviews in health care (CRD 2009). Using an “integrative evidence approach” is especially appropriate for this review since ECT is a complex procedure, bound with stigma and controversy. The integrative methodological approach of this review will integrate qualitative data with quantitative data through four phases, as illustrated in Figure 1. The first phase will begin with qualitative literature scoping and data analysis in order to inform protocol development, next there is quantitative and qualitative data extraction and analysis, and finally the integration of quantitative and qualitative findings into a final interpretation and conclusion. The review will proceed in the following four phases (Figure 1): PHASE 1: QUALITATIVE LITERATURE SCOPING 1. Search for literature (qualitative literature scoping). 2. Identify all qualitative studies among the literature returned and determine whether they meet the pre-determined inclusion criteria. 3. Conduct critical appraisal of the qualitative studies. 4. Extract data according to pre-determined extraction forms. 5. Analyse qualitative evidence. PHASE 2: REFINEMENT OF PROTOCOL 1. Refine the review questions. 2. Refine the PICO components. PHASE 3: ANALYSE QUANTITATIVE & QUALITATIVE DATA 1. Screen all quantitative studies according to revised protocol. 2. Conduct critical appraisal of the quantitative studies. 3. Extract data according to outcomes of interest. 4. Conduct analyses (of qualitative and quantitative data). PHASE 4: INTEGRATE QUALITATIVE FINDINGS WITH QUANTITATIVE FINDINGS 1. Integrate quantitative and qualitative data together. 2. Write report. Appendix 2. The Integrative Methodological Approach, phase 1 and 2 (Refinement of Protocol) Qualitative literature scoping and refinement of the protocol (phase 1 and 2) The extensive literature scope identified 64 studies, all references were uploaded, screened and data extracted in the DistillerSR online systematic review program (www.evidencepartners.com). We read 53 articles in full text. Only five studies met our pre-defined inclusion criteria for data extraction (Johnstone 1999; Koopowitz 2003; Orr 2005; Rajkumar 2007; Smith 2009) and the qualitative assessment and review instrument, JBI-QARI (JBI 2007) was therefore not used. We used a simplified content analysis approach to identify key concepts in qualitative studies where patient experiences may help to enhance understanding of clinical outcomes. Three reviewers (RB,KAL,BH) independently used a data recording form (Appendix 7) to identify text units directly pertaining to the emotional impact of the location/setting, intervention, outcome (e.g. experience of memory loss). We then determined the frequencies of these categories. Two reviewers (RB, KAL) identified prominent and recurring Electroconvulsive therapy for depression (Protocol) Copyright © 2011 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd. 19 concepts in the articles and agreed on a set of categories for the data extraction form. Each reviewer independently used the dominant categories to form suggestions for refinement of the protocol. We then drafted and discussed a list of strategies to refine the protocol until consensus was reached. Given the limited amount of data from the four included studies consensus was easily reached. Based on the available qualitative evidence, we designed suggestions for refinement in two PICO element areas: (P) population and (O) outcome. With respect to P, gender and (legal) compulsion emerged as dominant categories. We suggested the addition of pre-specified sub-group analyses for i) gender and ii) voluntary vs forced (involuntary) treatment conditions. Regarding O, the three categories i) anxiety, ii) distress (perception of coercion/trauma), and iii) cognitive abilities on both short and long term scale emerged as dominant categories. With respect to the effectiveness review, our suggestion for protocol refinement was to add short term (less than six months after ECT) cognitive abilities and supplement the secondary outcomes, with validated scales measuring anxiety, such as (Hamilton Rating Scale for Anxiety (Ham-A) (Hamilton 1959), Beck Anxiety Inventory (BAI) (Beck 1988), Clinical Anxiety Scale (CAS) (Snaith 1982) and distress such as the Impact of Event Scale (IES) (Horowitz 1979). Appendix 3. Included and excluded diagnoses Appendix Table 1. Included and excluded diagnoses Included DSM-IV code Major Depressive Disorder 296.xx Major Depressive Episode** Disorder, Single 296.2x ICD-10 code F32.x Major Depressive Disoder, Recurrent 296.3x F33.x Depressive Disorder NOS* 311 F32.9 Mood Disorder NOS* 296.90 F39 Mood Disorder Due to... [Indicate the 293.83 General medical Condition] - with depressive features, with major depressive-like episode or with mixed features F06.32, F06.33 Excluded Dysthymic Disorder 300.4 F34.1 Bipolar I Disorder, Single Manic Episode 296.0x F30.x Bipolar I Disorder, Most recent Episode 296.4x Hypomanic F31.x Bipolar I Disorder, Most Recent Episode 296.5x Depressed F31.x Electroconvulsive therapy for depression (Protocol) Copyright © 2011 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd. 20 (Continued) Bipolar I Disorder, Most Recent Episode 296.6x Mixed F31.6 Bipolar I Disorder, Most Recent Episode 296.7 Unspecified F31.9 Bipolar II Disorder (Recurrent Major 296.89 Depressive Episodes with Hypomanic Episodes) F31.8 Bipolar Disorder NOS* 296.80 F31.9 Substance Induced Mood Disorder 291.8, 292.84 *NOS Not Otherwise Specified Appendix 4. QUALITATIVE full text screening form Questions for including and excluding full text QUAL articles 1. Are the reported empirical QUAL data (data including quotes provided by participant(s), i.e. not only author/researcher interpretation) from a primary study? [Qual or mixed - mixed methods studies that incorporate both quantitative and qualitative components may be included, but the qualitative component of the study is subject to the same inclusion criteria as the mono-methods studies] • Yes • No • Unclear 2. Are the study participants’ adults (>15 yrs) who have received ECT for depressive illness? • Yes • No • Unclear 3. Is the focus participants’ views on received ECT treatment? [Including but not limited to: knowledge, attitudes, beliefs, perceptions, awareness, understandings] • Yes • No • Unclear 4. Did the researchers ascertain first hand experiential accounts on participant’s views about ECT treatment by asking directly about ECT? • Yes • No • Unclear 5. Were the data collected through interviews or focus groups, and were these either recorded and transcribed or were extensive notes taken? • Yes • No • Unclear 6. Were the data analysed in a qualitative way? [e.g. phenomenology, narrative, hermeneutics, thematic, etc] Electroconvulsive therapy for depression (Protocol) Copyright © 2011 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd. 21 • Yes • No • Unclear 7. Decision (after filling in the form) • Unclear • Include (All questions are answered “YES”) • Exclude (Not all questions are “YES”) 8. Reason for exclusion (main question number):......................... 9. Comments: ........................................................................................................................................... Appendix 5. MEDLINE search strategy 1. Electroconvulsive Therapy/ 2. (electroconvulsive$ or electr$ convulsive$).tw. 3. (electroshock$ or electro-shock$).tw. 4. ect.tw. 5. or/1-4 6. mood disorders/ 7. mood disorder$.tw. 8. exp Affective Disorders, Psychotic/ 9. affective.tw. 10. bipolar.tw. 11. (manic$ or mania$ or hypomani$ or hypo-mani$).tw. 12. Depressive Disorder/ 13. Depression, Postpartum/ 14. Depressive Disorder, Major/ 15. Seasonal Affective Disorder/ 16. (depression$ or depressed$ or depressive$).tw. 17. melanchol$.tw. 18. or/6-17 19. 5 and 18 20. randomized controlled trial.pt. 21. controlled clinical trial.pt. 22. randomized.ab. 23. placebo.ab. 24. randomly.ab. 25. trial.ab. 26. groups.ab. 27. 20 or 21 or 22 or 23 or 24 or 25 or 26 28. Qualitative research/ 29. Nursing methodology research/ 30. (qualitative adj3 (research$ or method$ or approach$ or study or studies or evaluation$)).tw. 31. (ethnon$ or emic$ or etic$ or ethnograph$ or ethnolog$ or hermeneutic$ or heidegger$ or husserl$ or colaizzi$ or giorgi or van kaam or van manen).tw. 32. (participant adj3 observ$).tw. 33. constant compar$.tw. 34. focus group$.tw. 35. grounded theory.tw. 36. narrative analys$.tw. Electroconvulsive therapy for depression (Protocol) Copyright © 2011 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd. 22 37. 38. 39. 40. 41. 42. 43. 44. 45. 46. 47. 48. 49. 50. 51. 52. 53. 54. 55. 56. 57. 58. 59. 60. 61. 62. 63. 64. 65. 66. 67. 68. 69. 70. 71. ((lived or life or patient$) adj experience$).tw. theoretical sampl$.tw. phenomenolog$.tw. Questionnaires/ exp Interviews as Topic/ (interview$ or questionnaire$).tw. or/28-42 exp case control studies/ exp cohort studies/ Comparative Study.pt. Cross-sectional studies/ Epidemiologic studies/ clinical trial/ or controlled clinical trial/ or multicenter study/ case control.tw. cohort.tw. cross sectional.tw. (epidemiologic adj2 study).tw. ((follow up or followup) adj2 study).tw. longitudinal.tw. observational.tw. (prospective adj2 study).tw. retrospective.tw. (exploratory or pilot).tw. or/44-59 case reports.pt. comment.pt. letter.pt. editorial.pt. or/61-64 60 not 65 19 and 27 19 and 43 19 and 66 humans.sh. (67 or 68 or 69) and 70 Appendix 6. CCDANCTR search strategies CCDAN’s Specialized Register (CCDANCTR) The Cochrane Depression, Anxiety and Neurosis Group (CCDAN) maintain two clinical trials registers at their editorial base in Bristol, UK, a references register and a studies based register. The CCDANCTR-References Register contains over 26,000 reports of trials in depression, anxiety and neurosis. Approximately 65% of these references have been tagged to individual, coded trials. The coded trials are held in the CCDANCTR-Studies Register and records are linked between the two registers through the use of unique Study ID tags. Coding of trials is based on the EU-Psi coding manual. Reports of trials for inclusion in the Group’s registers are collated from routine (weekly), generic searches of MEDLINE, EMBASE and PsycINFO; quarterly searches of the Cochrane Central Register of Controlled Trials (CENTRAL) and review specific searches of additional databases. Reports of trials are also sourced from international trials registers c/o the World Health Organisation’s trials portal (ICTRP), drug companies, the hand-searching of key journals, conference proceedings and other (non-Cochrane) systematic reviews and meta-analyses. Details of CCDAN’s generic search strategies can be found in the ‘Specialized Register’ section of the Cochrane Depression, Anxiety and Neurosis Group’s module text. The CCDANCTR will be searched as follows: CCDANCTR-Studies Register Electroconvulsive therapy for depression (Protocol) Copyright © 2011 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd. 23 Search : CONDITION = (depress* or dysthymi* or “adjustment disorder*” or “mood disorder*” or “affective disorder*” or “affective symptoms”) AND INTERVENTION = (“electroconvulsive therapy” or ECT or electrotherapy or “electric stimulation” or electroacupuncture or electroacupuncture or “vagal nerve stimulation”) CCDAN-CTR-References Register Search: Free-text=(depress* or dysthymi* or “adjustment disorder*” or “mood disorder*” or “affective disorder*“ or ”affective symptoms“) and (ECT or electroconvuls* or electro-convuls* or electroshock or electro-shock or (electro* and acupuncture) or convuls* or (deep* and brain*) or ((vagus* or vagal) and nerve*)) Appendix 7. QUALITATIVE data extraction form QUALITATIVE data extraction [For protocol refinement and to inform the effectiveness review] I. Assessment of study characteristics A. Publication/study 1. Study identifier (first author year or study name year - e.g. Rose 2001.................. 2. Publication year (e.g. 1983) ................ 3. Publication type • journal article • brief communication • conference abstract • book • book chapter • dissertation • other……………………………………………………… 4. Year(s) of data collection (e.g.1983; 1997 to 1999)................... 5. Country (state/city) where study was undertaken and data collected......................... 6. Study setting • inpatient hospital/clinic • outpatient clinic, community centre • participants home • other……………………………………..…. B. What is known about the main researcher (first author)? 7. Researchers demographic data: Age ............yrs Gender: F/M............Unknown/not reported................ Other researcher data:............................................................................................................................... 8. Researchers disciplinary background: • psychologist • physician, psychiatrist • nurse • student • unknown, not reported • other……………………………….…………… 9. Other researcher information source of funding.................................................................................................................................... other researcher data.............................................................................................................................. C. The research question/aim/purpose/objective of the study? 10. What is the main research question/aim/objective (use words in text) ................................................................................................................................................................. ................................................................................................................................................................. Electroconvulsive therapy for depression (Protocol) Copyright © 2011 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd. 24 11. Secondary or other research question/aim/objective (use words in text) ................................................................................................................................................................ ................................................................................................................................................................ D. Characteristics of study population 12. Number of participants.............................................................. 13. Age - mean/median or range (in years).................................... 14. Gender - % female (e.g. 20.4).................................................. 15. Ethnicity • White or Caucasian • Hispanic or Latino • Black or African American • Asian • other…………………………… • not reported 16. Diagnostic information (e.g. ICD-10/DSM-IV diagnosis): ................................................................................................................................................................ ................................................................................................................................................................ 17. Other population details (state briefly): ................................................................................................................................................................ ..............................………………………………………………………………………………………………… E. What is known about the ECT intervention (type, number given etc) 18. Type of ECT • modified (with anaesthesia) • not-modified (without anaesthesia) • unilateral • bilateral • brief pulse wave • sine wave • unknown type, comments…………………………………………………….. 19. Number of ECT series/courses and total number of ECTs given (e.g. in mean or range) ................................................................................................................................................................ 20. Report of response to ECT treatment (e.g. complete recovery, no response) ................................................................................................................................................................ 21. Comments and other information about the ECT intervention ................................................................................................................................................................ ................................................................................................................................................................ F. Study type and findings 22. Data collection (methods) - briefly describe e.g. sample selection (random, by convenience, language preference, contactability, interview capacity etc), length of interview(s), nr of interviews etc ................................................................................................................................................................ 23. Analyses (methods) - briefly describe if interviews audiotaped, transcribed (how by whom), documentation and analyses process/ use of audit trail etc ................................................................................................................................................................ ................................................................................................................................................................ 24. Main findings (list and use words in text) ................................................................................................................................................................ ................................................................................................................................................................ 25. Was the data also analysed quantitatively? • Yes • No II. Extraction of study findings and data Electroconvulsive therapy for depression (Protocol) Copyright © 2011 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd. 25 Does the study data indicate that we should consider refining aspects of the effectiveness review related to 26. A. location/setting? (e.g. subgroup analyses) If YES, explain why (interpretation): ................................................................................................................................................................ 27. Quotes from text about A. location/setting (separate quotes with numbers, 1) 2) etc) ................................................................................................................................................................ ................................................................................................................................................................ 28. B. participant characteristics (P)? If YES, explain why (interpretation): ................................................................................................................................................................ 29. Quotes from text about B. participant characteristics (P) ................................................................................................................................................................ ................................................................................................................................................................ 30. C. the intervention (I) (i.e. ECT type, unmodified, modified brief pulse/sine wave, unilateral/bilateral etc) If YES, explain why: ................................................................................................................................................................ 31. Quotes from text pertaining to C. the intervention (I) ................................................................................................................................................................ ................................................................................................................................................................ 32. D. comparisons (C)? If YES, explain why: ................................................................................................................................................................ 33. Quotes from text pertaining to D. comparisons( C ) ................................................................................................................................................................ ................................................................................................................................................................ 34. E. outcomes (O)? If YES, explain why: ................................................................................................................................................................ 35. Quotes from text pertaining to E. outcomes (O) ................................................................................................................................................................ ................................................................................................................................................................ 36. F. our research questions (see protocol)? If YES, explain why: ................................................................................................................................................................ 37. Quotes from text pertaining to F. our research questions ................................................................................................................................................................ ................................................................................................................................................................ 38.III. General and other comments ................................................................................................................................................................ ................................................................................................................................................................ Appendix 8. Risk of Bias for non-RCTs (cognitive function) III. Risk of Bias (RoB) for non-RCT studies (cognitive function) ITEM If groups, METHOD of ALLOCATION JUDGEMENT DESCRIPTION (YES=Low RoB, UNCLEAR=Insufficient data to judge, NO= High RoB) • YES • UNCLEAR • NO A priori List of confounders Considered: (considered important and de• YES fined in ECT review protocol) • UNCLEAR Controlled for : • YES • UNCLEAR Electroconvulsive therapy for depression (Protocol) Copyright © 2011 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd. 26 (Continued) • NO • NO AGE Considered: • YES • UNCLEAR • NO Controlled for : • YES • UNCLEAR • NO GENDER Considered: • YES • UNCLEAR • NO Controlled for : • YES • UNCLEAR • NO MARITAL status Considered: • YES • UNCLEAR • NO Controlled for : • YES • UNCLEAR • NO SOCIOECONOMIC status Considered: • YES • UNCLEAR • NO Controlled for : • YES • UNCLEAR • NO LIFE EVENTS Considered: • YES • UNCLEAR • NO Controlled for : • YES • UNCLEAR • NO OTHER (comorbid) PHYSI- Considered: CAL ILLNESS • YES • UNCLEAR • NO Controlled for : • YES • UNCLEAR • NO OTHER Considered: • YES • UNCLEAR • NO Controlled for : • YES • UNCLEAR • NO ITEM JUDGEMENT BLINDING? INCOMPLETE addressed? DESCRIPTION • YES • UNCLEAR • NO data • YES • UNCLEAR • NO Free of SELECTIVE reporting? • YES • UNCLEAR • NO Electroconvulsive therapy for depression (Protocol) Copyright © 2011 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd. 27 (Continued) Free of OTHER bias (specify)? • YES • UNCLEAR • NO A priori PROTOCOL? • YES • UNCLEAR • NO A priori ANALYSES plan? • YES • UNCLEAR • NO HISTORY Protocol first published: Issue 5, 2011 CONTRIBUTIONS OF AUTHORS Leiknes (KAL) and Høie (BH) originated the idea for the protocol. KAL, Berg (RB), Smedslund (GS) wrote the protocol. KAL has contributed to all phases of the protocol development. Øverland (SØ) contributed to an early initial protocol draft written by KAL. Hammerstrøm (KTH) designed the extensive research strategy. RB has designed Integrative Methodological Approach as described in the protocol. GS has contributed to the quantitative methodological section. Jarosch-von Schweder (LJS) has contributed to all clinical matters, as well as background, research questions, diagnostic considerations and intervention parameters. BH has contributed to issues related to neuropsychological tests and cognitive functioning. KAL, RB and BH have extracted and analyzed the qualitative data and given suggestions for refinement of the protocol, according to the Integrative Methodological Approach. KAL has revised the protocol. All reviewers have contributed actively to the protocol proposal, participated in discussions and helped clarify questions and provided suggestions for overall amendments. DECLARATIONS OF INTEREST None known SOURCES OF SUPPORT Electroconvulsive therapy for depression (Protocol) Copyright © 2011 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd. 28 Internal sources • Norwegian Directoriate of Health, Norway. Financial support of the work undertaken in the Norwegian Knowledge Centre by the Norwegian Directorate of Health External sources • No sources of support supplied Electroconvulsive therapy for depression (Protocol) Copyright © 2011 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd. 29