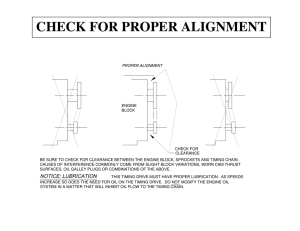
Heat Engines
advertisement

Review of Work and PV diagrams Since , the force that the gas exerts on the piston is given by F = PA . Then the work done by the gas during the expansion is given by: This area is positive for positive work done by the gas (corresponding to gas expanding and +ΔV) This area is negative for negative work done on the gas (corresponding to gas compressing and -ΔV) Heat Engines For a constant mass of gas, the operation of a heat engine is a repeating cycle and its PV diagram will be a closed figure. If the cycle is operated clockwise on the diagram, the engine uses heat to do net work. If operated counterclockwise, it uses work to transport heat and is therefore acting as a refrigerator or a heat pump. The Thermodynamic System •In a thermodynamic cycle, energy is applied in one form to change the state of the system, and energy is then extracted in a different form to return the system to its original state. •In a heat engine, the energy is applied in the form of heat to change the state of a working fluid and then extracted in the form of mechanical work to return the working fluid to its initial state. From the Gas Laws PV = kNT •Putting energy in the form of heat into a gas will increase T, but at the same time the gas laws mean that the gas P or V or both must increase in proportion. •The gas can be restored to its original state by taking this energy out again but not necessarily in the form of heat. •ΔP or ΔV can be used to perform work by moving a suitably designed mechanical device such as a piston or a turbine blade. •The greater the ΔT, the more energy which can be extracted from the fluid. A heat engine is a system that performs the conversion of heat or thermal energy to mechanical work. (1) Heat is absorbed from a source at a high temperature (2) Work is done by the engine (3) Heat is expelled by the engine at a lower temperature Heat Engines Heat Engines Heat engines enable heat energy to be converted to kinetic energy through the medium of a working fluid. Heat is transferred from the source, through working fluid in the heat engine and into the sink, and in this process some of the heat is converted into work. Heat engine theory concerns only the process of converting heat into mechanical energy, not the method of providing the heat, the combustion process. Combustion is a separate conversion process and is subject to its own efficiency losses. Heat Engines The work done by the entine equals the net heat flowing into the engine: W = Qh – Qc The net work done for cyclic process is the are enclosed by the curve representing the process on a PV diagram. The thermal efficiency of an engine, Eff, is the ratio of the net work done to the heat absorbed. Eff= 1 – Qc/Qh Th is the temperature of the heat source; Qh is the heat absorbed Tc is the temperature of the cold reservoir; Qc is the heat given off Energy conversion process Whether it is coal, oil, gas or nuclear power, 80% of the world’s electricity is derived from heat sources. Almost all of the energy conversion processes convert thermal energy into electrical energy and involve an intermediate step of converting the heat energy to mechanical energy in some form of heat engine. •First Steam engine was developed in 1769 2nd Law of Thermodynamics “Heat will not flow spontaneously from a colder body to a warmer body AND heat energy cannot be transformed completely into mechanical work.” The bottom line: 1) Heat always flows from a hot body to a cold body 2) Nothing is 100% efficient What the 2nd law of Thermodynamic does to our lovely heat engine. . . . Nothing is perfect! It is impossible to construct a heat engine that is 100% efficient. The best conversion efficiency achieved today is only around 60% for combined cycle steam and gas turbine systems. Efficiencies in the range of 35% to 45% are more common for steam turbines, 20% to 30% for piston engines and as low as 3% for OTEC ocean thermal power plants. This is why we say: Laws of Thermodynamics (paraphrased): (1) You can't win; you can only break even (conservation of energy - you won’t get out more work than you put in) (2) Rule (1) lied; you can’t even break even. Example Problem 13.30 An engine absorbs 1700 J from a hot reservoir and expels 1200 J to a cold reservoir in each cycle. (a) What is the efficiency of the engine? (b) How much work is done in each cycle? (c) What is the power output of the engine if each cycle last for 0.3 sec? Example Problem 13.30 An engine absorbs 1700 J from a hot reservoir and expels 1200 J to a cold reservoir in each cycle. (a) What is the efficiency of the engine? Example Problem 13.30 An engine absorbs 1700 J from a hot reservoir and expels 1200 J to a cold reservoir in each cycle. (b) How much work is done in each cycle? Example Problem 13.30 An engine absorbs 1700 J from a hot reservoir and expels 1200 J to a cold reservoir in each cycle. (c) What is the power output of the engine if each cycle last for 0.3 sec?