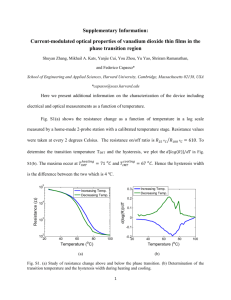
as a PDF
advertisement

Spectral reflectance of the human ocular fundus Frangois C. Delori and Kent P. Pflibsen Reflectance spectra from discrete sites in the human ocular fundus were measured with an experimental reflectometer in the visible and near-infrared parts of the spectrum. The principal study population consisted of ten subjects 22 to 38 years of age withba wide range of degree of fundus melanin pigmentation. Reflectance spectra were obtained from the nasal fundus, the fovea, and an area 2.5°from the fovea. Spectra were also recorded from several older subjects and from one aphakic patient with a coloboma. The reflectance spectra were found to be influenced by the degree of individual and local melanin pigmentation of the fundus, the amount of blood in the choroid, the transmission properties of the ocular media, and the discrete reflections in the stratified fundus layers. Mathematical models of the optical properties of the stratified layers are proposed and are fitted to the experimental fundus reflectance spectra. The models account for the absorption by blood, melanin, macular pigment, and ocular media, and incorporate tissue scattering and discrete reflectors corresponding to anatomical layers. I. Introduction The interaction between light and intraocular tissues plays an important role in the application and interpretation of optical methods for diagnosing and treating ocular disease. Fundus photography, ophthalmoscopy, fluorescein angiography, psychophysical testing, photocoagulation, and several noninvasive optical diagnostic methods are dependent on and, to various degrees, affected by the absorption or scattering characteristics of tissues in the stratified layers of the fundus. As these methods become more refined, it is important to gain a better understanding of light interaction with the fundus layers. Fundus reflectometry has been used to investigate the optical properties of the fundus,1-10 to study the dynamics of visual pigments,1 1- 13 to obtain quantitative and qualitative information on the choroidal1 4 -17 and retinal1 8 circulation, to optimize photocoagulation,19 and to measure the amount of ocular melanin2 0 and macular pigment.2 1 22 Differences in spectral reflectance from various fundus sites are the basis of color observation of the fundus and have been exploited in monochromatic photography.2 3 24 Although these studies have provided useful results on specific The authors are with Eye Research Institute of Retina Foundation, Biomedical Physics Unit, 20 Staniford Street, Boston, Massachusetts 02114. Received 5 July 1988. 0003-6935/89/061061-17$02.00/0. © 1989 Optical Society of America. entities, they have not yielded detailed information on spectral characteristics of fundus reflectance and on the influence of melanin pigmentation. This may be due to the limited spectral range and resolution used in most previous studies and to the smallness of populations studied. Furthermore, the interrelated effects of absorption by pigments and reflection or scattering by tissues have not been systematically analyzed. This paper presents measurements of fundus reflectance from 450 to 800 nm from discrete fundus areas in normal subjects with a wide range of fundus melanin pigmentation. The influence of the degree of fundus melanin pigmentation and age on the reflectance spectra is examined in detail. A first level of analysis considers the effect of the individual ocular absorbers and reflectors on fundus reflectance characteristics. A second level combines information on the individual absorption and scattering components into optical (mathematical) models of the fundus layers and fits these models to the experimental reflectance data. A model proposed by van Norren and Tiemeijer1 0 was evaluated and a new model, incorporating added complexity, was proposed and assessed. II. Experimental Methods A. Fundus Reflectometer and Data Analysis The experimental fundus reflectometer used in this study is based on a modified Carl Zeiss fundus camera (Fig. 1). Illumination for observation and focusing is provided by lamp TL (maximum retinal irradiance: 7 mW cm- 2 , 5-min maximal permissible exposure time2 5' 26). Polychromatic illumination for reflectance measurements is provided by 3-ms flashes of the xenon 15 March 1989 / Vol. 28, No. 6 / APPLIEDOPTICS 1061 dent as far as light safety calculations are concerned.2 5 The resulting spectrum Sf,xwas recorded by means of a monochromator digital printer. A reference spectrum S was ob- tained after each fundus measurement by measuring the light reflected from a barium sulfate surface (diffuse reflector, 96 ± 3% reflectance, 400-1200 nm), lo- cated 155 mm from the entrance pupil of the camera (adjusted to be in the subject's pupil plane). If the focal length of the eye is assumed to be 22 mm, the equivalent reflectance of this reference is 0.96(22/155)2 = 1.9%. The reference spectrum was recorded with the same flash power setting as used for fundus measurements and with the same illumination and sampling apertures. Light intensities for successive flashes were constant within 5%. The spectral reflectance R, of the fundus was calculated for each wavelength Xby R = 0.019 Fig. 1. Diagram of the experimental fundus reflectometer. See text for explanation of symbols. Unmarked components are standard optical parts of the fundus camera. arc lamp FL (maximum retinal irradiance: 70 mJ cm-2 , 36%of the maximum permissible exposure2 5' 26). An aperture FA, located in the illumination beam in a plane conjugate to the retina, limits the illumination to a circular fundus area of -5° in angular diameter (visual angle). The light reflected by the fundus is imaged by the camera optics in the plane of a diaphragm MA. The latter is initially open (6°), allowing the observer to focus and to align the camera, via mirror SM, on the fundus area of interest. The diaphragm is then closed to define the sampling area of 1-4° in diameter. The diameter of the sampling area is noted for each measurement from a graduated scale visible through the eyepiece. Rotation of SM activates the xenon flash, and the light sampled by MA is imaged by lens R on a fiber optic LF. The input face of this fiber is located in a plane conjugate to the entrance pupil of the optical system, and its output face, which is slit-shaped, serves as the entrance slit to a grating monochromator. The intensity distribution in the dispersed spectrum is measured simultaneously at all wavelengths by a Vidicon camera used in conjunction with a multichannel analyzer (Princeton Applied Research). This system allows integration and storage of reflected light intensities on 512 wavelength channels. The spectral range of the reflectometer system is 400-912 nm (700-1212 nm after monochromator adjustment). The effective spectral resolution of the system is 7.5 nm. Two or three spectra from one fundus site were generally accumulated in the instrument's memory to improve the signal-to-noise ratio. These repeated measurements were recorded with at least 5-s intervals to allow for alignment and can therefore be considered indepen1062 APPLIEDOPTICS / Vol. 28, No. 6 / 15 March 1989 n, S, ) (1) where n and nr are the number of flashes used to record the fundus and reference spectra, respectively. Each reflectance spectrum was plotted as log reflectance vs wavelength. For each spectrum, reflectances at twenty selected wavelengths (listed in Table I) were tabulated for statistical analysis and curve fitting using RSE software (BBN Research System). The calculated fundus reflectances represent total fundus reflectance (integrated over all angular directions) only if the eye had perfectly transparent media, a focal length of 22 mm, and a perfectly diffuse reflecting fundus. Although corrections could be made to account for media transmission2 7 and for focal length differences, it is not possible to measure the angular distribution of the reflected light and therefore determine which fraction of the reflected light is collected by the entrance pupil of the reflectometer. Because of Table 1. Average Fundus Reflectance for Ten Subjects at Three Different Sites Equivalent reflectance in % Wavelength (coefficient of variation in %) (nm) Nasal fundus Perifovea Fovea 445 455 480 505 522 540 548 560 565 569 575 586 595 610 640 675 705 728 750 805 1.01 (18) 1.14 (14) 1.44 (13) 1.82 (23) 1.94 (24) 1.88 (18) 2.04 (20) 2.25 (26) 2.27 (26) 2.24 (23) 2.20 (21) 2.72 (33) 4.17 (44) 6.36 (47) 8.46 (46) 10.21 (43) 11.28 (40) 11.82 (38) 11.94 (36) 12.52 (30) 0.68 (32) 0.73 (31) 0.89 (31) 1.20 (26) 1.41 (23) 1.44 (22) 1.53 (23) 1.61 (22) 1.61 (22) 1.61 (22) 1.62 (22) 1.88 (23) 2.62 (34) 3.99 (49) 5.79 (55) 7.70 (53) 8.93 (49) 9.71 (46) 10.42 (43) 11.27 (36) 0.23 (29) 0.25 (30) 0.33 (30) 0.58 (26) 0.94 (18) 1.11 (14) 1.18 (13) 1.24 (14) 1.26 (15) 1.28 (15) 1.29 (16) 1.48 (18) 2.11 (30) 3.30 (49) 4.97 (54) 6.68 (52) 7.83 (50) 8.63 (46) 9.30 (44) 10.37 (38) these limitations, we refer to our measurements as "equivalent reflectances." M= E 2 (2) - RmodX), WX(RObsX n1 1i0 where n is the number of wavelengths (n = 19), and Wx B. Subjects and Measurement Sites Our principal study population was composed of ten normal subjects ranging in age from 22 to 38 years and with no ocular abnormalities. Two subjects were Black and eight were Caucasian. Among the latter, two subjects had brown irises, three hazel or green irises, and three blue irises. The pupil of one eye of each subject was dilated to a diameter of at least 6 mm with 1% benzeneacetamide (Tropicamide). The subject's head was stabilized on the chin rest of the cam- is a weight assigned to each wavelength measurement. We used WA = Robs,X-1 as a compromise between equally weighting absolute errors at different wavelengths (Wx = 1) and equally weighting relative errors (WA = Robs,- 2 ).30 For each completed fit, we calculated the standard error associated with each parameter, the F/ratio, the statistical significance, and a relative error (RE in %) defined as RE = 100 [(1/n) era. Reflectance spectra were also recorded from sev- eral older subjects (age 60-65 years) and from one aphakic patient (age 74 years) with a coloboma in one eye. Reflectance measurements were recorded for the ten young subjects from the nasal fundus, the perifovea, and the fovea. These sites were selected because they present substantial differences in anatomy and in pigment content. Spectra were also recorded from the optic disk, and those results will be reported in another communication.2 8 In this study, the illumination area was always 5 in angular diameter. For the nasal fundus, the measurement was made (nf = 1-2) at about 1 disk diameter from the disk edge, in an area devoid of large retinal vessels, with a sampling area of 3-4 in diameter (fixation: subject looking at a fixation target with fellow eye). The sampling aperture used nasally was larger than that used for the other sites to minimize the variability associated with nonuniformities in the nasal fundus. For the perifovea, a measurement consisted of the average of four measurements (nf = 4) obtained at 2.50 from the fovea in the horizontal and vertical directions, with a sampling area of 1.2-1.6 in diameter (fixation: subject looking at top, bottom, left, and right side of the illuminated area with measurement eye). For the fovea, the measurement (nf = 2-3) was made centered on the fovea, with the same sampling area as for the perifovea (fixation: subject looking at center of illuminated area using a crosshair with open intersection). C. Curve-Fitting Procedure Two mathematical models representing the optical properties of the fundus layers are analyzed in this study (see Sec. V). In each case, the reflectance predicted by the model (RmodX) was represented by a complex function of unknown parameters. The observed fundus reflectances (Robs,0) at nineteen wavelengths (listed in Table I, except for 445 nm) were fitted to the Rmod,X function using the curve-fitting procedure CURFIT described by Bevington.2 9 This very efficient algorithm makes a least-squares fit to a function, which may be nonlinear in its parameters, using a combination of a gradient search with an analytical solution developed from linearizing the function. The unknown parameters were found by minimizing a figure of merit M defined as . E (R0 bSX- RmodX)2/Robs X (3) To characterize the goodness of the regressions for all spectra at each of the three sites, we used two criteria. First, we calculated a pooled relative error (PRE in %) given by E I ~~~~~~~~~~~~~~1 F PRE = 100 [(/ns) (R.,.,, - Rmod,) /Robs,X (4) where s is the number of spectra at one site (s = 10). Second, we computed the average F/ratios (AFR) of the regression for all spectra at one site. Ill. Fundus Reflectance Spectra A. Experimental Results Reflectance spectra from the nasal fundus (N), the perifovea (P), and the fovea (F) for the ten young subjects are shown in Figs. 2(a) and (b). Table I gives, for the twenty selected wavelengths, the average reflectances and coefficients of variation for the three sites. Figure 3 (top) shows spectra recorded at different sites between the arcuate bundle and the macula in one subject to illustrate changes in reflectance spectra with fundus location. Figure 3 (bottom) shows the reflectance spectra recorded at a choroidal nevus and in the area surrounding that nevus to demonstrate the effect of a marked variation in choroidal melanin pigmentation. Fundus reflectance at all sites was always lowest at the shortest wavelength measured (445 nm) and highest at long wavelengths (>640 nm). Measurements on two subjects for wavelengths >800 nm (results not shown) revealed a pronounced reflectance minimum centered at 790 nm, a maximum around 1070 nm, and no reflectance above 1200 nm. These characteristics correspond with the absorption spectrum of water in the ocular media. Similarly, the slight irregularities observed in the spectra around 760 nm (Figs. 2 and 3) may correspond with a weak absorption band of water centered at that wavelength. As the degree of melanin pigmentation increases, the wavelength of maximal reflectance lengthens and the reflectance decreases. This reduction is more pronounced in red than in green light: the ratio of the reflectance of the lightest fundus (subject 1, Fig. 2) and that of the darkest fundus (subject 10) is -6 at 675 nm, but only -1.7 at 575 nm. Similarly, the coefficients of variation associated with 15 March 1989 / Vol. 28, No. 6 / APPLIEDOPTICS 1063 100. ........................................ (a) 80 1 2 3 4 5 a) C) F N a' NN N P F N P F 20 P F P N a) N LL a: / 1.0 -a P~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~ NI P N P N N~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~ a) F F 03 0. 2 450 F 550 650 750 450 550 650 750 450 1 . 550 650 750 450 S50 650 750 450 550 650 750 Wavelength (nm) 100 l(b). 6 7 8 9 10 a) N F p N P ._ U) N a, F N ~~~~~~P P F C UCL 1.0 N N N P P N N ,P P ~~~~~~~~~~~~P I a, F 0U F 0.1 450 550 50 750) 450) 550 i50 750 450 F 550 650 750 450 F 550 r650 750 450 550 650 750 Wavelength (nm) Fig. 2. Reflectance spectra from the nasal fundus (N), the perifovea (P), and the fovea (F) in ten subjects (1-10) with different degrees of ocular melanin pigmentation. Subjects 1-8 were Caucasians with blue irises (1,2,3), green or hazel irises (4,5,6), brown irises (7,8), and subjects 9 and 10were Black. The illumination area was 50 in diameter in all cases, and the sampling area was -4° for the nasal data and 1.6° for the macular data. the mean reflectance (Table I) are substantially larger for red light (45-55%) than for blue and green light (13-26%). An extreme example of the lower dependence of green light reflectance on the choroidal melanin concentration is that of the nevus (Fig. 3, N and S): the green light reflectances are about equal for the nevus (N) and the surrounding area (S), but the red light reflectances are different. All spectra reveal to various degrees the absorption characteristics of oxyhemoglobin (Fig. 4). Distinct reflection minima or inflections in the spectra at 540 and 575 nm correspond to the absorption maxima of oxyhemoglobin at those wavelengths, and the pronounced increase in the reflectance for wavelengths longer than 575 nm corresponds to the dramatic decrease in hemoglobin absorption in that spectral range. With increased ocular pigmentation there is a marked reduction of the prominence of the hemoglobin absorption bands at 540 and 575 nm. The reflectance maximum at 560 nm, seen clearly in the lightly pigmented fundus, and the increase in reflectance above 1064 APPLIEDOPTICS / Vol. 28, No. 6 / 15 March 1989 575 nm become gradually less pronounced as the de- gree of pigmentation increases. The flattening of the blood spectra observed with increased degree of individual melanin pigmentation is also seen with changes in local pigmentation between the nasal (Fig. 2, N) and macular (P and F) sites, and between a site above the arcuate bundle (Fig. 3, spectrum 1) and the macula (spectrum 5). The hemoglobin absorption bands of the macular spectra show distinct flattening, even in the lightly pigmented eyes where the reflectance maximum at 560 nm is barely resolved. The sudden increase in reflectance for wavelengths longer than 575 nm is less marked than nasally, but is nevertheless always detected even in the darkest fundi investigated (subjects 9 and 10). Two-way analysis of variance (three sites, ten subjects) performed separately for each wavelength of Table I showed that fundus reflectance was significantly (p < 0.05) affected by choice of site for all wavelengths, and by subject for wavelengths longer than 575 nm. This confirms that variation in degree of I (0= 10 0 2 4 5 .0 c 10 - 0) 5U)Z a) C a) °I 0() 0) 0 S N C: a) 0 I C Ca 0 0 Hb 1.0- 0 0 C.)D C L Cr c~ x aw S N 0. I 400 500 600 700 8600 Wavelength(nm) Fig. 3. Top, reflectance spectra from subject 2 for an area superior to the arcuate bundle (spectrum 1), for an area centered on the fovea (spectrum 5), and for three equally spaced areas between the previous sites (spectra 2-4). Bottom, reflectance spectra from subject 4 in an area centered on a choroidal nevus (N) and from an area adjacent to the nevus (S). These spectra were offset by a factor of 10 toward lower reflectances to avoid overlap with the other spectra. The reflectance in the 530-580-nm range was slightly higher for N (nm) Wavelength Fig. 4. Absorption characteristics of the ocular pigments. HbO 2 , 9 oxygenated hemoglobin, data from van Assendelft3 ; right scale, extinction coefficients of hemoglobin with an oxygen saturation of 15 mg/100 mliter). Data were 95% (hemoglobin concentration: ME, melaaveraged over the 7-nm bandwidth of the reflectometer. 4 3 nin, data from Gabel et al.3 and Menon et al.3 (solid line) and from Geeraets et al.3 5 (interrupted line), representing extremes in the rate of spectral dependence; relative scale. MP, macular pigment, data from Snodderly et al. 44 ; left scale, approximate optical density in humans. optical density for the standard observer. than for S. Examination of fundus photographs showed slight alteration in the RPE in the area of the nevus. Both sets of spectra were obtained with an illumination aperture of 50 and a sampling area of -4°. Pairpigmentation is mainly manifested in red light. wise comparisons via the Scheffe method3 l indicated further that, with a confidence of 95%,the reflectance of the nasal fundus was significantly higher than that of the perifovea (455 < X < 725) and that the reflectance of the perifovea was significantly higher than that of the fovea (455 < X< 575). The latter difference is most marked for X < 520 nm (Fig. 2) and is caused by the absorption by the macular pigment in the fovea. B. Comparison with Other Studies Reflectances of the human fundus are affected by variations in the degree of fundus pigmentation and by variations related to the fundus site measured. Hence it is difficult to accurately compare our results with those obtained in other studies,1,6"0,2 0 ,21 often on a single site in only a few subjects. Comparison is fur- ther complicated by differences in reflectometry methods, particularly with regard to reflectance references, spectral resolution, area of sampling, and illumination field. Best agreement between different studies appears to occur for the green spectral range, especially with regard to foveal measurements. However, most studies, with the exception of that of Hunold and Malessa, 2 0 do not reveal the spectral detail seen here, particularly with regard to the hemoglobin absorption bands. This results from the larger num- 7 OM, ocular media, data of van Norren and Vos2 ; left scale, ber of wavelengths recorded by Hunold's and our technique which allow better resolution of the hemoglobin spectral bands. Variability of results among studies is largest for red light measurements. This may be due to the inherent large variability of red light reflectance associated with variation in degree of melanin pigmentation (Table I). Results for wavelengths shorter than 500 nm are also more variable than those in green light. Our data correspond well with those of Brindley and Willmer, 2 ' Charman, 8 and van Norren and Tiemeijer,10 but the other studies 6 20 report larger reflectances. This appears to be related to a larger contribu- tion from backscattering in the ocular media associated with the larger illumination areas used in the latter studies. Indeed, for constant retinal irradiance, light density in the lens and hence the amount of backscattered light, increases with the area of illumination. IV. Optical Constituents of the Fundus Interpretation of the reflectance spectra requires a working knowledge of the absorption properties of the various pigments and of the reflection and scattering properties of the different anatomical layers of the fundus. Analysis of the spectra is aided by distinct spectral signatures of some pigments (oxyhemoglobin, macular pigment), but complicated when the absorption and/or scattering properties vary monotonically throughout the spectrum (melanin, ocular media, and tissue scattering). We discuss the optical properties 15 March 1989 / Vol. 28, No. 6 / APPLIEDOPTICS 1065 of various constituents of the fundus layers and relate those with observed reflectance characteristics. 0.37 t 0.20 D.U. The RPE density showed no racial dependence. 3 6 One can estimate, from the highest RPE density measured (0.8 D.U.),3 3 that the extinction A. coefficient of melanin Ocular Pigments Fundus reflectance characteristics are strongly influenced by the absorption of light by blood throughout the fundus, by melanin pigment in the choroid and retinal pigmented epithelium (RPE), by macular pigment in the fovea, and by the ocular media. Absorption by visual pigments does not affect the present reflectance data since more than 99.5% of the photopigments are bleached by the observation light (6.8 32 log troland units). To facilitate initial interpretation of our results, we use the Lambert-Beers law to derive information on different pigments. We assume that all the light incident on the fundus is transmitted by all pigments, reflected by a posteriorly located reflector with reflectance Rb,x\, retransmitted by the pigments, and detected by the reflectometer. tance RA is given by logRx = logRb 6 In that case, fundus reflec- - Kp - dp 2 Kpa Dp,x (5) p where Kpx is the absolute extinction coefficient for each pigment (p), dp is the single-pass path length, K' are the relative extinction coefficients normalized at X, and DpAn is the single-pass optical density at a normalizing wavelength X. The highly simplified fundus reflectance model, described by Eq. (5), will be referred to as model I. The degree of melanin pigmentation of the fundus appears to be the most important variable affecting magnitude and shape of the reflectance spectra. The absorption spectrum of melanin is generally found to decrease monotonically with increasing wavelength throughout the visible spectrum. Measurement by Gabel et al. 3 3 on RPE melanin and by Menon et al. 3 4 on iris melanin showed a -4.6 spectral dependence of the absorption coefficient around 550 nm (ME, solid curve, Fig. 4). Other studies showed flatter spectra, with the absorption spectra recorded by Geeraets et al. 35 (ME, interrupted line, Fig. 4) demonstrating the weakest dependence (X-2.2). In vitro studies by Weiter et al.3 6 of melanin pigmentation have shown that, for a population of Caucasian and Black subjects, the optical density (500 nm) of choroidal melanin at the posterior pole varies between 0.2 and 4 D.U. (Density Units),3 7 with a marked racial dependence. Fundus reflectance, assuming double passage of light through the choroid, does not reveal this large density range, indicating substantial flattening of the melanin absorption spectrum. The optical density (500 nm) of the RPE at the posterior pole was found by Weiter et 36 3 1066 (Caucasian), and brown (Black) irises, respectively. The Spearman's p were 0.94 (p < 0.0001), 0.75 (p < 0.02), and 0.82 (p < 0.004) for the nasal fundus, perifovea, and fovea, respectively. The correlations between iris color and the amount of melanin in the defined) always showed negative p. However, these correlations were statistically significant only in the 595-750-nm range for each site and in the 480-505-nm range for the nasal fundus (low absorption by blood). 2. Blood (Hemoglobin) Absorption of light by blood occurs primarily in the highly vascularized choroid. The contribution of reti- nal capillaries is small as they occupy a volume of 0. 15 gt liter cm- 2 of fundus3 8 or an equivalent blood layer -1.5,gm thick. Melanin to be 0.27 ranked as 1, 2, 3, and 4 for blue, green or hazel, brown choroid are expected since choroid and iris are part of = logRb,\ - 2 al. is at least 800 cm- 1 (as- the same anatomical layer (the uvea). Rank correlations between iris color and fundus reflectances at wavelengths other than 675 nm (where the P index is p 1. Kme,500 suming -10-um thick RPE cells). To characterize fundus melanin pigmentation, we used, as did Hunold and Malessa,2 0 a melanin pigmentation index defined as P index = -ogR 6 7 5 . Absorption by choroidal blood is very low at 675 nm and ocular media transmission is highest (Fig. 4). Under the assumption of Eq. (5), the P index is linearly related to the amount of melanin. The P indices of the nasal fundus, perifovea, and fovea correlate significantly with each other (all p < 0.001). The P indices for the three sites also correlate significantly with iris color, 0.08 D.U., 7 and by Gabel et al.33 to be APPLIEDOPTICS / Vol. 28, No. 6 / 15 March 1989 The average oxygen saturation of cho- roidal blood is high, because the arteriovenous oxygen saturation difference in the highly perfused choroid is small. As a result, the absorption spectrum of choroidal blood is essentially related to that of oxyhemoglobin (Fig.4).39 The blood volume in the human choroid is not known with accuracy. Comparison of the ultrasonographically determined choroidal thickness in vivo, 350-450 m,40 41 with the thickness in enucleated eyes, 200-250,gm, 4 2 allows one to estimate that 30-60% of the choroid is occupied by blood, in the assumption that vascular collapse is the primary reason for the difference in thicknesses. 41 Interpretation of the blood signature on the reflectance spectra is facilitated by considering a particular property of the oxyhemoglobin absorption spectrum, which is that light absorption by oxyhemoglobin is approximately equal at 455, 540, and 575 nm (Fig. 4). The extinction coefficients Kb at these wavelengths, when averaged over the spectral bandwidth of the reflectometer, are within 5% of each other, and this equality is maintained at other oxygen saturations. Figure 5 shows how this property is used to associate with any fundus reflectance spectrum, a hypothetical spectrum R, for which the extinction coefficient Kb of blood would be a constant. The slope of the logRA expected amount of blood in the choroid. This is especially true if one considers the sole contribution of the choriocapillaris, which forms a quasicontinuous vascular sheet, -10 ,m thick.4 3 If reflection from the fundus involved reflection from the choroid only, one would expect the reflectance spectra to at least show the spectral signature of the choriocapillaris. This may be the case for lightly pigmented fundi using the 610-nm estimate of dhb, but not for darker fundi and CO IDI LL for all values of the 560-nm estimate. 540 455 t575 560 610 Wavelength(nm) Fig. 5. Schematic reflectance spectrum from the fundus with the The RXspectrum drawn through the hypothetical spectrum R. 455-, 540-, and 575-nm points of the real spectrum (logRx) always shows a slight negative curvature. The logR* spectrum represents fundus reflectance if blood were replaced by a spectrally neutral absorber with extinction coefficientKb (= Khb,455 = Khb,540 = Khb,575, see Fig. 4). This spectrum is influenced by melanin absorption, ocular media transmission, etc., but not by spectral changes in blood absorption. Because of the low curvature of logR, one can assume that logR< varies linearly with X beween 540 and 610 nm, and logRx can then be calculated by intra- or extrapolation along a line through the 540- and 575-nm points. The differences, at 560 and 610 nm, between the logRx and the logR; spectra are used in Eq. (6) to estimate the amount of blood responsible for the hemoglobin spec- tral signature on the fundus spectra. spectrum at -550 nm was not significantly different for the three sites and was on average 0.17 J 0.06 log reflectance units per 100 nm (all sites, n = 30). To estimate the amount of blood sampled by reflec- tometry, it is possible to calculate the thickness of an equivalent layer of blood that would be needed to account for the observed spectral signature of blood on the fundus spectra. Assuming that all the incident light traverses this blood layer twice with reflection by deeper layers, we use Eq. (5) for logRx (extinction coefficient Khb,x) and for logR' (extinction coefficient Khb). The reflectance of the deeper layers and the contribution of all nonblood terms can then be eliminated, and the blood layer thickness dhb (arterial blood) is then derived as logR,\ dhb - 2 - We can con- clude that reflectometry samples the choroid only partially, that a substantial amount of light is reflected anterior to the choriocapillaris, and that light penetration in the choroid is less pronounced at 560 nm than at 610 nm. A reflector must be located anterior to the choroid (Sec. IV.B.2), and light transmitted by this reflecting layer must, in darkly pigmented fundi, be strongly absorbed in the choroidal stroma. 3. MacularPigment (Xanthophyll) The macular pigment is located in the inner retinal layers and extends over an area of 0.5-2.0° in diameter, centered on the fovea.2 4 44 The spectral signature of this pigment, with its high absorption in blue light (Fig. 4),44 is recognized in most subjects (Fig. 2) by the fact that foveal reflectance Rf, is lower than the perifoveal reflectance Rp,\ for X < 520 nm. An estimate of the macular pigment density can be obtained by comparing the foveal reflectance to the perifoveal one. Following Brindley and Willmer2 l and van Norren and Tiemeijer,1 0 we assume that all the light reflected from the fovea is reflected by the deeper layers with double transmission through the macular pigment. Applying Eq. (5) for the fovea (reflectance Rfx, macular pigment density Dmp,460) and for the perifovea (reflectance Rpx,assuming no macular pigment), after subtraction one finds log f,\ = log RP,A ' R ,A 2 -D K' PX (7) mp4 where R >x and Rpx are the reflectances of the deeper layers at the fovea and perifovea, respectively. KmpX is the extinction coefficient of the macular pigment, normalized at 460 nm (Fig. 4). For each subject, the data at 445, 455, 480, 505, 522, 540, and 548 nm were fitted to Eq. (7), assuming that the ratio Rcx/Rp,, logR~ (KhbX - K4b) (6) The thickness dhb was calculated for X = 560 and 610 nm (Table II), with logR' computed as indicated in Fig. 5. The significant decrease in dhbwith P index demondecrease in the apstrates a pigmentation-dependent Table II. Equivalent BloodLayer Thickness Calculated with Eq. (6) at 560 and 610 nm for Three Sites inTen Subjects Wavelength Equivalent blood layer Linear correlation thickness dhb of dhb with the P index parent amount of blood sampled by reflectometry. (nm) Site in /im Equivalent blood layer thicknesses dhbare substantially higher for the estimation at 610 nm than at 560 nm, 560 Nasal fundus 3.9 ± 2.7 -0.73 Perifovea Fovea 2.1 i 0.8 1.5 ± 0.7 -0.50 -0.08 n.s. n.s. 6.1 -0.90 p < 0.0004 indicating that the simple model (in which all the reflected light traverses the blood layer) cannot be reconciled with fundus reflectance spectra. The absolute values of the equivalent blood layer thickness (Table II) are very small compared to the 610 Nasal fundus 14.9 Perifovea Fovea 12.7 ± 6.0 12.6 ± 6.4 + p <0.02 -0.92 p < 0.0002 -0.94 p < 0.0001 15 March 1989 / Vol. 28, No. 6 / APPLIEDOPTICS 1067 (which accounts for reflectance difference in the absence of macular pigment) was wavelength independent. For the ten subjects, Dmp,460was found to vary . . . . . . . . 1001. Sc between 0.12 and 0.32 D.U., with a mean of 0.19 ± 0.06 D.U. (single pass). The factor log(Rf \/Rp,\) was 0.10 CO + 0.08,and all regressions were statistically significant (worst case: p < 0.02). The individual Dmp,460 results c for absorption by the macular pigment. a: will be used in the optical model (Sec. V.A) to account 4. Ocular Media Equivalent reflectances are affected by absorption in the ocular media, which are traversed by both the incident and reflected light. Transmission through the media of young subjects increases rapidly from 400 to 550 nm and reaches a constant value of -80% above 45 650 nm. ing contribution depends on field size and can be estimated to be 0.1 D.U. (80%transmission) for the aperture sizes used here. 4 6 With age, there is a decrease in the transmission of the media and an increase of the slope of the transmission spectrum. 4 5 This is seen in Fig. 6 for the spectra recorded from the nasal fundus of two older subjects (N60 and N64): a faster decrease in reflectance with decreasing wavelength is observed compared to young subjects. Indeed, the slope of logR\ spectrum (Fig. 5) was 0.30 and 0.34 log reflectance units per 100 nm, compared with 0.17 + 0.06 for the young subjects (Sec. IV.A.2). The spectrum of the aphakic eye (Fig. 6, N74) had a slope of 0.11, smaller than those of the young subjects, indicating that the crystalline lens is the major contributor to ocular media absorption and scattering. Thus, ocular media have an important influence on measured fundus spectra of older subjects; however, they are not expected to play a major role for the young subjects in this study. Ocular Reflectors 1. Sclera The sclera is often considered the most important reflector in the fundus. Smith and Stein4 7 measured its reflectance on enucleated eyes as 50-70%at 675 nm. Alpern et al.4 8 measured scleral reflectance in three subjects with ocular colobomas as 30% at 675 nm. Our measurement in an aphakic subject with a coloboma (Fig. 6, CO) also indicated an equivalent reflec- tance of transmission 33% at 675 nm. With an ocular media of 70-80%, this would correspond to a scleral reflectance of 40-50%. The scleral reflectance decreases slightly with increasing wavelength, as seen for the coloboma (Fig. 6, CO) and from measurements of the scleral reflectance at the conjunctiva (Fig. 6, 1068 10- 0 N64 cn cC CO cc cc C. w I- UJ Van Norren and Vos27 compiled literature data on media density in young subjects (ages 20-30), and derived an optical density spectrum for a standard observer (Fig. 4). They showed that these densities correspond to subject-dependent light absorption in the media, and that a wavelength-independent contribution must be added to the absorption term to account for light scattering in the media. This scatter- B. CO N60 N74 APPLIEDOPTICS / Vol. 28, No. 6 / 15 March 1989 10. ! I ! 400 ! 500 1 i 600 700 I I 800 4 Wavelength(nm) Fig. 6. Reflectance spectra from the nasal fundus (N) in three subjects with age as indicated. The 74-yr old subject was aphakic; the other two subjects were phakic. CO,reflectance spectrum from a coloboma in the 74-yr old aphakic subject. These measurements were obtained with an illumination aperture of 5 and a sampling area of 40. SC, reflectance from the sclera at the conjunctiva in a young subject (2). This measurement is relative as no absolute reference was used in this instance. The absorption bands of blood from the conjunctival capillaries are clearly seen; the interrupted line is the R spectrum associated with the SC spectrum (see Sec. IV.A.2). These spectra demonstrate that scleral reflectance decreases continuously with increasing wavelength. SC). The latter reflectance was found to decrease by a factor of 2.2 ± 0.6 between 500 and 800 nm (four young subjects). 2. Reflectorsin the Retinal Layers Reflections by the deeper retinal layers have been shown in separate experiments to originate from layers between the photoreceptors and Bruch's membrane,2 from layers between the photoreceptors and the choriocapillaris,17 from the RPE or sites close to the RPE,5 7, and from the photoreceptors.3' 9 Interpretation of monochromatic fundus photographs2 3 2 4 also supports the existence of reflections originating at the level of the RPE. The analysis of our spectra, using the argument involving the spectral signature of the choriocapillaris (Sec. IV.A.2), argues for the existence of one or several reflecting layers anterior to the choriocapillaris. For green wavelengths, these layers reflect a substantial amount of light resulting in a poor sampling of the choroidal space by the incident light. The poor penetration of green light in the choroid was also strikingly demonstrated by the spectra of the nevus (Fig. 3, S and N), which show the inability of green light reflectance measurements to detect the increased melanin concentration in the choroid. The photograph of Fig. 7 clearly demonstrates the presence of a reflecting layer, by the fact that distinct (c) (b) (a) RX om e . phr rpe\ bm - 1111 I I IIII E D.. Do., D~mp r ji D. Dmp D Dpc Dine cc *x D.e -_ dhb chs S. dhb D,, l rM SC - Model III Model II of the fundus layers: (a) Schematic representation Fig. 8. rSt om, ocular media; im, inner limiting membrane; phr, photoreceptors; rpe, retinal pigmented ephithelium; bm, Bruch's membrane; cc, choriocapillaris; chs, choroidal stroma; and sc, sclera. (b) and (c) Diagrams for model II and model III, respectively. Parameters with an asterisk are fixed; the others are adjusted by curve fitting in each model. The D symbols represent single-pass densities, r, reflec- tances, and d, blood layer thicknesses. Fig. 7. Fundus photograph obtained using oblique illumination of the fundus. An illuminating fiber optics was applied on the conjunctiva. The angle of incidence of light at the posterior pole of the eye was estimated to be 30°, and the projected direction is indicated by an arrow. The distance between the vessel and its shadow is 149 ± 10 ,tm (average of sixteen sites). The shadow is formed on a surface located -140/tan (300) or 240 Am posterior to the vessels. shadows of the retinal vessels can be observed when the fundus is obliquely illuminated. The shadows are formed on a surface capable of localized reflection and located -240 gm posterior to the retinal vessels and thus at the level of the RPE or the anterior choroid. A located near similar demonstration of a reflecting layer the RPE was given by Mori et al.4 9 by projecting through the pupil a very fine slit of light obliquely on the fundus. Detection of the reflections at a different angle demonstrates two discrete reflections: one orig- inates at the limiting membrane and the other from a to the limiting membrane (at layer 280 gm posterior 50 2.50 from the fovea). Specular reflections from the inner limiting membrane are easily detected by ophthalmoscopy (especially in darkly pigmented fundi), and several studies have suggested that the limiting membrane is the principal origin of retinal reflections.4 5 7 The intensity of this reflection depends critically on curvature and orientation of the retinal surface, on direction of the incident light, and on position of the entrance pupil of the detecting system.9 V. Optical Models of the Fundus Each fundus layer has different absorption and scattering properties [Fig.8(a)]. If the anterior layers can be considered anatomically well organized and hence optically relatively homogeneous (with the exception of large retinal vessels which could be avoided during reflectometry), this is not the case for the deeper lay- ers. The choroid is composed of blood vessels, melanocytes, and other scattering bodies distributed in a nonhomogeneous fashion. Explicit modeling of each constituent of the fundus strata is an intractable problem. Instead, we propose models based on simplifying assumptions and will evaluate how well they describe the experimental results. By gradually increasing the complexity of the models and by including optical properties from the literature, we have attempted to explain the interrelationship between reflectance spectra and the optical properties of the fundus layers. The simplest reflectance model for the fundus layers is one where all the incident light is transmitted by the retinal and choroidal layers, reflected by the sclera, and retransmitted by the- choroid and retina [Eq. (5), model I]. However, as discussed in Sec. IV.B.2, reflec- tions originating from the retinal layers contribute substantially to the overall fundus reflectance, especially at short wavelengths and for darkly pigmented fundi. A model for fundus reflectance, incorporating such a retinal reflecting layer, was proposed by van Norren and Tiemeijero and tested on experimental reflectance data of four subjects. We evaluate this model (model II) as a description of our experimental reflectance spectra. A. Fundus Reflectance Model 11 1. Descriptionand Parametersof ModelII Model II [Fig.8(b)] consists of two spectrally neutral reflectors, the sclera (reflectance rsC)and an anterior reflector (located anterior to the RPE but posterior to the macular pigment in the fovea, reflectance rpe), which sandwich all the blood and melanin of the fundus. A blood layer (thickness dhb) simulates choroidal blood, and a melanin layer (density Dme,500)simulates melanin in the RPE and choroid. The model also accounts for absorption by the macular pigment 15 March 1989 / Vol. 28, No. 6 / APPLIEDOPTICS 1069 the five remaining parameters were calculated by fitting Eq. (8) to the experimental data using the curve- r fitting procedure of Sec. II.C. 2. Results of Regressions for Model II Figure 9 presents the regression results of model II for three individual spectra, and Table III gives the average model parameters at the three sites. The fits were always highly significant (p < 0.0001) and generally tighter for moderate pigmentation. The nasal fits were on average better than the macular ones (PRE, Table III). The fine detail of the hemoglobin absorption bands in the 500-600-nm range was generally not 0.2 a) Dmne - 1.26(0.05) C) , .' c ID a) ' 11 , , " .dhb ,, ,/ - 33 (17) Dom - 0.76 (0.06) ,. , , well fitted (Ni, Fig. 9). - p,,e----- 2.3(0.. L Examination of the correlation coefficients indicates that the model parameters show pronounced 1517 E trends, statistically significant in many cases, to be negatively correlated with the amount of melanin. Since these relationships do not correspond with our expectations, based on the anatomical evidence, we must conclude that model II is less than optimal at describing the optical properties of the fundus layers. In particular, the inverse correlation between the amount of blood and melanin, which strongly affected ~~~~~~~~~~~~Dom ~~~~~~~~~~~rpe model I (Table II), is reduced but still present in model ~~~~~~~~~~~rsc -8.9 (1.1) : ~~~~~~~~~~~RE II despite the introduction of rpe. ~~~~~~~~~~~~F-ratio Because of these interrelationships, it is difficult to comment on the results obtained for the various parsc - 17.7 (0.6) a), RE-4.4 DC g /F-ratio C . LlW.1i/- Dme l I ,,,,,,,.~~~..........,,:,,,,,, i am Zf f / D. - 21 f (0.2) j 4(9 - 044 (0.07)-, 1.3 :"' X X h.......... 43)......... dh a (0. 1) . -4.1 / 2100 Table Ill. , ADO 500 600~~~Bo 700 BOO Parameter 900 Dme, 500 Wavelength Unit D.U. (Melanin) (nm) Fig. 9. Model II results: experimental reflectance data ()and fitted regression (solid lines) for three spectra; N, nasal fundus of dhb rp, and RE. The interrupted lines are spectra reconstructed from (a) reflectance contri- bution of the anterior reflector seen through the ocular media (and macular pigment) and (b) reflectance of the choroid in the absence of the anterior reflector. (Dmp,460) and the ocular media (Dwn,420).The absorption spectra for the four pigments are given in Fig. 4, in absolute terms for blood (Khbx)and in normalized terms for other pigments (KA). Application of the Lambert-Beer law gives the reflectance R correspond- ,lm (Hemoglobin) subject 1; P7, perifovea of 7; and F10, fovea of 10. The regression results (S.E. in parentheses) are given for each spectrum. The units are D.U. for D,, D, and D p; m for db; and percent reflectance for the model results in the following conditions: Resultsof Regressionsfor Model Iia Dom,420 (Ocular media) D.U. 10 2(KmADm,420 +Kmp,ADmp4a) [rp + (1 -re)rsc X 10 2(KmeDme,50 + Khsdhb)] (8) six unknown parameters. The macular pigment densities were assumed zero for the nasal and perifoveal data, and the values for Dmp,460, obtained in Sec. IV.A.3, were used for the foveal data. 1070 The values of APPLIEDOPTICS / Vol. 28, No. 6 / 15 March 1989 Fovea 0.64 (57) [0.00-1.24] 1.09 (51) [0.45-2.171 1.16 (50) [0.51-2.20] 93 (61) [21-204] r = -0.63 p = 0.05 111 (58) [30-216] r = -0.58 n.s. 99 (58) [32-1863 r = -0.74 p < 0.02 0.51 (37) [0.15-0.87] r = -0.66 0.65 (17) [0.48-0.82] r = -0.12 0.66 (21) [0.43-0.93] r = -0.33 p <0.04 n.s. n.s. 1.7 (15) [1-3-2.1] r = -0.59 n.s. % 2.7 (33) [1.6-4.9] r = -0.80 p < 0.006 2.1 (21) [1.5-2.9] r = -0.59 n.s. rsc (Sclera) % 14 (35) [6-22] 16 (31) [8-25] PRE AFR a where Dom,420,Dme,500 Dmp,460,dhb, rc, and rpe are the Perifovea rpe (RPE reflector) ing to Fig. 8(b): Rmod., = Nasal fundus % - 15 (33) [8.9-24.5] r =-0.37 r = -0.75 r = -0.73 n.s. p < 0.02 p < 0.02 3.52 4254 4.03 3706 4.58 4188 See text for explanation of symbols. For each group of data, bold numbers indicate the mean of ten subjects; parentheses indicate coefficient of variation; square brackets indicate range; r represents the linear correlation coefficient of the parameter with Dme,500; and p represents the statistical significance of this correlation (p = 0.05 attained with Irl = 0.63, through each pigment. n = 10). The densities are single pass rameters. Although the mean dhb results are reasonable (90-110 gim, -25% of the volume of a 400-um choroid), the dhb obtained for high pigmentation (-20 corresponds ,gm) are clearly too low. The mean Dom,420 well with the standard observer data of van Norren and Vos.2 7 The scleral reflectance r for all spectra (6- 25%) are low compared to the expected values (4060%), as discussed in Sec. IV.B.1. Comparison of our results with those obtained by van Norren and Tiemeijero are complicated by the strong dependences on degree of melanin pigmentation. Their four Caucasian subjects appeared to have been fairly darkly pig- mented compared with our subjects, as judged from the shape of the published spectra and from the reflectance data at 675 nm. With this in mind and within the limitations of the comparison, our values correspond well with those obtained in van Norren's study. 3. Limitations of Model II The anterior reflector rpeis efficient at flattening the hemoglobin absorption bands in the 500-575-nm range but has little effect at longer wavelengths because of its low relative magnitude. Thus, the ob- served reflectance changes in the 575-650-nm spectral range are principally fitted by dhb (similar to the model I, 610-nm estimate of Sec. IV.A.2). Large changes in Robsx for light pigmented fundi are therefore matched by large dhb whereas the more attenuated changes for dark fundi are fitted by lower dhb, resulting in the observed correlation between dhb and Dne500 (Table III). An attenuation of the hemoglobin spectrum at high melanin pigmentation would reduce this interde- B. Fundus Reflectance Model II We modified model II to include a light scattering contribution in the choroid. Light scattering in a pigmented tissue limits the depth of penetration of light and causes light to be reflected back toward the detector from various depths within the tissue. The resulting reduced path lengths through the pigments decrease net absorption and weaken the influence of the absorption bands. We also introduced several fixed parameters corresponding to specific anatomic layers and values chosen consistent with the literature. Furthermore, we reduced the number of adjustable model parameters and chose these to best represent the anatomical quantities expected to vary among individuals. We fixed the density of the ocular media and the reflectance of the sclera, because these parameters are not expected to vary greatly among young healthy subjects. To offset the resulting constraint, we introduced a single parameter under the form of an unknown density (D,) and located this source of light loss in the layer for which we have the largest uncertainties, i.e., the choroid. From analysis of D. behavior, we hoped to learn more about the imperfection of the model, without the confounding influence of several interdependent parameters. 1. Descriptionand Parametersof Model III Model III [Fig. 8(c)] consists of a scleral reflector, an absorbing-scattering layer simulating the choroidal stroma, a blood layer simulating the choriocapillaris (thickness d,,), a melanin layer simulating the RPE and a spectrally neutral anterior re(density Drpe,500), pendence of dhb and Dme,50 flector (reflectance rpe). The model also accounts for The fit in the 640-805-nm range is essentially de- absorption by the macular pigment (density Dmp,460) and r,, since the other pigments and rpe and the ocular media, but neglects the contribution of fined by Dme,500 have little effect in that range. The slope of logRmod,x the retinal capillaries (Sec. IV.A.2). The reflection at is thus that of (logr, - 2K'mexDme5OO).When Dme,500 the limiting membrane was not implicitly included in decreases and becomes steeper. This increases, Rmod,X the model and can be thought as being part of rpe for increase in slope is apparently too pronounced for the the nasal fundus and perifovea. In Sec. V.B.5, we slope of Robs,x,forcing r,, to decrease and Dmeto adopt a lower value. This interaction is the origin of the nega- tive correlation between rc and Dme(Table III) and would be diminished if the effect of melanin spectrum was weakened. The low values of r,, obtained in this and the van Norren and Tiemeijer study1 0 result partially from the fact that transmission of the ocular media in the 640-805-nm range was assumed to be 100% (Fig. 4). In fact, media transmission at those wavelengths is -80% (Sec. IV.A.4), and accounting for this in Eq. (8) causes an increase in the fitted r,, value. In conclusion, the effect of absorption by hemoglo- bin and melanin is too pronounced in model II. Introduction of light scattering within the choroidal space would result in an attenuation of the influence of these pigments. The anterior reflector coarsely flattens the hemoglobin bands in the middle of the spectrum but is unable to influence the reflectances at long wavelengths. Finally, although the use of six parameters (including Dmp, 460) generally provided tight regressions, too many parameters could contribute to the interparameter correlations observed in this model. investigate separately the influence of rilm on the mac- ular pigment density in the fovea. For light losses in the ocular media, we maintain the absorption component of model II (density DO,,420)but introduce a wavelength-independent scattering term (density Dms),as discussed in Sec. V.A.3. Application of the Lambert- Beer law gives the reflectance RAcorresponding to Fig. 8(c): R-.d A= 10 mpxDmp,460+Dms) 2(K.,D.., 420+K [rpe+ (1 - re)rch X 10 2(KmeDrpeO5O+KhbXdc)] (9) The absorption spectra for the pigments are those given in Fig. 4, in absolute terms for blood (Khb) and in normalized terms for other pigments (K'm 'K'mp, and Kme,) The reflectance r~h is that of all the layers posterior to the choriocapillaris, or the choroidal stroma backed by the sclera (reflectance r The cho- roidal stroma is simulated by a homogeneous scattering layer in which hemoglobin and melanin are uniformly distributed. Mathematical solutions describing light reflectance by such layers were derived 15 March 1989 / Vol. 28, No. 6 / APPLIEDOPTICS 1071 by Kubelka and Munk,51 and have offered a simple means for quantitative treatment of absorption and scattering in biologicaltissues.52 5 3 The reflectance rch for the choroidal stroma backed by the sclera (reflectance r,,x) is given by the Kubelka-Munk equations51 : (1 - r')(a - b coth bSth)(10) rh-a + b coth bStch - rS,,'(10 with b = (a2 - 1)1/2 and (D.e, 500K'me,X+ Khb, dhb + D,) log1 Oe a1+ e, hbIhb(1 1) Stch where tch is the thickness of the choroid, and S is the bulk scattering coefficient (in cm-') of the homogeneous absorber-scatterer layer. For clarity, the notations used in model II have been maintained. The blood layer thickness dhb represents the amount of blood (fractional volume = dhb/t~h), and Dme,500 represents the amount of melanin in the choroid (fractional volume not known with accuracy). The wavelengthindependent parameter D. can be considered as a neu- tral absorber in the choroid or any other source of light loss from that space. Several parameters were kept permanently constant. The choroidal thickness th was 400 gim,the thickness of the choriocapillaris d was 10 gtm (Sec. IV.A.2),and the ocular media scattering term Dm, was 0.1 D.U. (Sec. IV.A.4). The scleral reflectance (in percent) was described by r,,x = 50 exp[-0.00261(X 675)], simulating the observed spectral dependence of the scleral reflectance (Sec. IV.B.1) and being 50% at 675 nm. Three other parameters were always equal for all sites in all subjects but were varied to optimize the quality of the fits at all sites. These parameters were the ocular media absorption density Dom,420, the scattering coefficient S, and the RPE density Drpe,500 The latter was initially equal at all sites but was then adjusted to match each site separately. Attempts to fit Drpe,500 together with Dine,500were not successful, as the two melanin signatures are too competitive in the curve-fitting procedure. The five remaining parameters Dme,500,dhb, D, rpe, and Dp, 460 were computed by fitting Eqs. (9)-(11) to the measured reflectance spectra, using the curve-fitting procedure of Sec. II.C. In model II, we used a macular pigment density Dmp,460 determined from foveal and perifoveal reflectances (Sec. IV.A.3). In model III, Dp, 460 was included in the curve fitting allowing an independent determination of the macular pigment density at all sites. 2. Selection of Parameters S, Dom,42o, and Drpe,500 Starting with Drpe,50o= 0.4 D.U., the approximate mean at the posterior pole from the study of Gabel et al., 3 3 we first adjusted combinations ofDom,420 and S to optimize the regressions by monitoring the average F/ratios (AFR) and the quality of the individual fits. With increasing S, we observed an improvement in the regressions, an increase in fitted values of dhb, Dme,500, and D, a flattening of the absorption bands of choroidal hemoglobin, and a decrease in rpe. The latter two 1072 APPLIEDOPTICS / Vol. 28, No. 6 / 15 March 1989 changes allowed for a tighter fit in the 500-600-nm spectral range (especially in lightly pigmented fundi). A broad optimum in AFRs was found for the combination S = 6 cm- 1 and Dom,420 0.5 D.U. A scattering coefficient S 6 cm-' means that the reflectance of the choroidal layer in the absence of a sclera and choroidal pigments is -20%, indicating that a substantial amount of light is now reflected from within the model choroid. Furthermore, the fitted value for dhb is 200 gim on average or -50% of the choroidal volume. Since the scattering coefficient of whole blood is -15 cm- 1 ,5 4 a half-filled choroid would have a scattering coefficient of -7.5 cm-', which is in reasonable agreement with our value of 6 cm-'. Our selected value Dom,420= 0.5 D.U. is smaller than the mean value of 0.6 D.U. for the standard observer data of van Norren and Vos.2 7 This difference might be justified in accounting for a contribution of stray light, which would effectively lower the media density. Since RPE densities vary for different areas of the fundus,3 3 36 , we next investigated the effect of changing Drpe,soo. The five model parameters were fitted to the reflectance data of the three sites for Drpe,500 values between 0.1 and 0.8 D.U. (Fig. 10). With increasing Drpe,500we observed a decrease in the amount of choroi- dal melanin Dre,500(the melanin signature on the spectra must remain the same), a decrease in dhb, an increase in rpe and in D, and no substantial change for Dmp,460.The changes in the average F/ratios (AFR) indicate that the regressions are on average optimal for Drpe,500= 0.2-0.4 D.U. at the nasal fundus, for Drpe50 = 0.3-0.6 D.U. at the perifovea, and for Drpe,500 = 0.5-0.7 D.U. at the fovea. Similar conclusions could be drawn from the changes in the PREs for the three sites. In vitro measurements by Weiter et al.3 6 indicated that the RPE density in the macular area (our foveal and perifoveal sites) is on average 1.6 times higher than at the posterior pole, and that the RPE density nasal to the disk (our nasal site) is on average equal or slightly smaller than at the posterior pole. A similar distribution of RPE melanin was found by Gabel et 33 al., who reported a mean value of 0.4 D.U. at the posterior pole (Sec. IV.A.1). We therefore selected a density Drpesoo = 0.35 D.U. for all nasal fundi. The density for the macular sites should then be -0.56 D.U. (1.6 times 0.35). Weiter et al.36 further showed that the distribution of choroidal melanin shows a broad maximum in the macular area. We can thus assume that the amount of choroidal melanin is the same for the perifovea and for the fovea (sites are 2.50 apart). Examination of Fig. 10 indicates that the model achieves this if Drpesooin the fovea is -0.1 D.U. larger than in the perifovea. We therefore selected a RPE density Drpe,500= 0.50 D.U. for the perifovea and 0.60 D.U. for the fovea. The three selected values for Drpes50o fall in the respective ranges of Drpe,500for which the regressions were optimal (Fig. 10). This does not necessarily prove that our choices are correct, but it is advantageous for achieving the best possible fits. Examination of the correlation coefficients indicates that the model III parameters, compared with model II, show reduced correlations with the amount 0.8 0.6 0.4 0.2 0.0 . . . . . . . . . . . . . . . 7000 6000 N -fN of melanin Dme 500 Although some trends, such as a 5000 tendency for rpeto decrease with increasing Dme,5oo,are 4000 3000 2000 P / F z F 1000 .4 .3 _e -2 P 5- 8 *1 N 43. N P 2. F - - 400 1* - 300 - o -200 F N E100 1 0 0.2 _D 0.1 5 the choroidal volume. The average quantity of choroidal melanin in the two Black subjects was 3-4 times xi F ~ F -c~-~ P ~ 0.3a F '_ne P .~~~~~~ -0.1 N ~~~~. 0.0 the reduction in the coefficient of variation associated with dhb (Tables III and IV). The large coefficient of variation associated with Dme5oo demonstrates the enhanced sensitivity of model III at detecting changes in the amount of melanin. The amount of choroidal melanin Dne,500 calculated from the model (Table IV) varies between 0.01 and 7.9 D.U. Although this range is larger than that measured 36 by in vitro measurements on enucleated eyes, it is not for estimate lower the unreasonable. Indeed, using Sec. cm-', (800 melanin of coefficient extinction the IV.A.1), the highest density of 7.9 D.U. represents at most a melanin layer of 99 ,um or no more than 25% of Ne 0.0 clearly detected, only 1 out of 12 correlations is statistically significant (DX with Dne,500for the fovea) compared with 6 out of 12 in model II. The absence of marked correlation between dhb and Dme,500is clearly an improvement over model II, especially considering 0.2 0.4 Drpe,50 . 0.6 . . . . - , 8 S CL Table IV. Results of Regressions for Model Ilila 0.0 Unit Nasal fundus Perifovea Fovea Drpe,500 D.U. 0.35 (fixed) 0.50 (fixed) 0.60 (fixed) Dme,500 (Melanin) D.U. 0.96 (109) [0.01-3.4] 1.92 (122) [0.22-6.4] 2.13 (139) [0.19-7.9] gm 146 (42) [65-2861 182 (48) [61-321] 168 (50) [60-304] Parameter I 0.8 (.U.) Fig. 10. Mean results for the average F/ratios (AFR) and for the five fitted parameters of model III, for different values of the RPE density Drpe,500. The symbolsN, P, and F (for nasal fundus, perifovea, and fovea, respectively) are located adjacent to the scale corresponding with each parameter. The filled squares indicate a significant (p < 0.05) correlation between Dme,50oand the parameter; open squares indicate no significant correlation. dhb (Hemoglobin) rpe (RPE reflector) % 3. Results of Regressions for Model III Figure 11 presents the regression results of model III for three spectra (same spectra as in Fig. 9), and Table IV gives the average model parameters for the three sites. The regressions of model III are always highly significant (p < 0.0001) and in general improved compared with those of model II (PRE and AFR, Tables III and IV). The relative errors RE [Eq. (3)] were de- creased by at least 10% for twenty-two out of thirty spectra (three sites). The improvement was most marked for the light and medium pigmented fundi, and a decrease in fit quality was observed in some dark fundi (Figs. 9 and 11, F10). The effect of scattering in the choroid can be seen from the much attenuated hemoglobin bands in the spectrum of choroidal reflec- tance (Figs. 9 and 11, curves b). The reflectance rpe seen through the ocular media (curves a) is relatively lower than in model II, allowing for a tighter fit for X < 600 nm. D.U. D, (Choroidal loss) Dmp,460 (Macular r=0.11 r=0.23 n.s. n.s. n.s. 3.7 (19) [2.8-5.21 2.9 (24) [2.2-4.2] 2.3 (16) [1.7-2.81 r = -0.62 r = -0.42 r =-0.35 n.s. n.s. n.s. 0.17 (58) [0.05-0.36] 0.09 (63) [0.00-0.15] 0.10 (79) [0.00-0.22] r =0.59 r =-0.27 r =-0.66 n.s. n.s. p < 0.04 D.U. -0.016 (208) 0.024 (117) [-0.08-0.0441 [-0.02-0.081 pigment) % PRE AFR r=0.12 - r = -0.43 r = -0.02 n.s. n.s. 2.74 6882 3.47 5245 a See text for explanation of symbols. 0.21 (26) [0.12-0.31] r = 0.52 . n.s. 4.65 6593 For each group of data, bold number indicate the mean often subjects; parentheses indicate coefficient of variation; square brackets indicate range; r represents and p the linear correlation coefficient of the parameter with Dme,500; represents the statistical significance of this correlation (p = 0.05 attained with Irl = 0.63, n = 10). The densities are single pass through each pigment. 15 March 1989 / Vol. 28, No. 6 / APPLIEDOPTICS 1073 roidal volume (thickness: dhb (42-49%) appears N1 . b Dme -0.00(0.01) dhb - 68 (4) rpe - 5.0. (0.0) D - 0.13 (0.01) Dmp - 0.04 (0.00) RE -4.7 F-ratio - 1124 ,.0 400 gim). The variability in large, especially for the macular sites. For the different subjects, the amount of blood dhb in the adjacent fovea and perifovea correlated with each other (r > 0.95, p < 0.0001), but no significant correlation was found between either of the macular sites and the nasal fundus (r = 0.44 for both combinations). The amount of macular pigment Dp,460 derived from the model (Table IV) is 0.21 0 P7 0. 1: Dme -1.5(0.1) dhb - 61 (10) rpe - 3.0 (03) Dx - 0.07 (0.02) Omp - 0.04 (0.03) RE - 4.4 F-ratio - 1522 a) U C a) U) crease results from the fact that a slight amount of macular pigment was detected at the perifovea. The Dmp,460 values found in this study are lower than the (n = 2 subjects) and by van Norren and Tiemeijer (n = 4), but higher than the 0.15 D.U. found by Alexander et al.22 (n = 5). However, the macular pigment densities measured by reflectometry are in general much lower than those determined by psychophysical meth- 20 ,j F10 LU f ........... *..-b Dme - 7.6 (0.0) .............. dhb - 126(35) ,. Dx -0.00 (0.02) Dmp - 0.31 (0.00) RE - 6.9 F-ratio -782 I. 402D 5OD 60 Wvplpnnth 60 70 00 nm Fig. 11. Model III results: experimental reflectance data (+) and fitted regression (solid lines) for three spectra: N1, nasal fundus of subject 1; P7, perifovea of 7; and F10, fovea of 10, The regression results (S.E. in parentheses) are given for each spectrum. The units are D.U. for De, D,, and Dmp;gm for dhb;and percent reflectance for rpe and RE. The interrupted lines are spectra reconstructed from the model results in the following conditions: (a) reflectance contri- bution of the anterior reflector seen through the ocular media (and macular pigment) and (b) reflectance of the choroid in the absence of the anterior reflector. higher than that in the darkest Caucasians. The lower melanin density in the nasal fundus corresponds with our ophthalmoscopic experience: choroidal vessels are often seen nasally in fundi that do not reveal any choroidal detail in the macular area, The amount of melanin De, 500 of the three sites correlated highly with each other (r > 0.93, p < 0.0001 for all three combinations). As expected, De,,500 also correlates significant- ly with the P index (Sec. IV.A.1) at each site (all r > 0.92, p < 0.0002). The amount of blood in the choroidal stroma dhb derived from model III (Table IV) ranges between 60 and 320 gim. Including the 10-,umcontribution of the choriocapillaris, this corresponds to 18-83%of the cho1074 al and perifoveal reflectances (Sec. IV.A.3). This in- average of 0.25 D.U. found by Brindley and Willmer 2 l C LL C 0.05 D.U. on average for the fovea (single pass), slightly higher than the...0.19 D.U. determined from the comparison of fove- APPLIEDOPTICS / Vol. 28, No. 6 / 15 March 1989 ods: Bone and Sparrock 5 5 found a mean density of 0.54 D.U. (460 nm) for a population of forty-nine subjects. Two factors could account for the differences between the reflectometric and psychophysical estimates. First, the size of the sampling field in reflectometry, 1.0-2.5° in the different studies, may be too large to resolve the narrow maximum in pigment density in the fovea.4 4 Second, the contribution of stray light and reflections at the limiting membrane (foveal reflex) would inevitably reduce the density measured by reflectometry. To investigate the influence of such reflection on the macular pigment density, we introduced a neutral reflectance rilm at the limiting membrane [Fig. 8(c)] and used our curve-fitting procedure to determine which value of rilm would cause the mean to increase to -0.5 D.U. For a reflectance rilm Dmp,460 = 0.8%, we found that the mean pigment density Dmp,460 became 0.51 0.22 D.U. Thus, small reflec- tions at the limiting membrane or small amounts of stray light have a pronounced effect on the measured pigment densities in blue light (low fundus reflectance). Finally, it is useful to compute the contribution of light reflected by the choroid and sclera [rch,Eq. (9)] to the total reflectance. This contribution was calculated using the model parameters obtained for each spectrum and correlated with the amount of choroidal melanin De,500. For the nasal fundus, rh contributes 615%of the total reflectance at 540 nm (r = -0.56, n.s.), and 50-80%at 675 nm (r =-0.91, p <0.0002). For the macular sites, rch is 2-8% of the total reflectance at 540 nm (r = 0.51, n.s.), and 40-90%at 675 nm (r = 0.96,p < 0.0001). Thus, light reflected by the choroid in blue and green light contributes only a small fraction of the total reflectance, especially in darkly pigmented fundi. It is also interesting to note that removal of the sclera (rS = 0) from the model reduces the total reflectance by only 1-4% in the darkest fundi at 675 nm and by 40- 55% in the lightest fundi. This corresponds well to 16 Thus, choexperimental measurements in rabbits. roidal scattering in the model (S) causes a substantial amount of light to be reflected from within the stroma, and the contribution of the scleral reflectance is very small in darkly pigmented eyes. 4. Limitations of Model III Model III is limited by its oversimplification of a complex biological tissue. As in any model, assump- tions must be made to reduce the number of parameters to be fitted by the model. In particular, our simulation of the choroidal stroma by a homogeneous absorbing-scattering layer is naive when one considers that choroidal vessels and melanocytes are irregularly distributed in the stroma. Choroidal vessels may be more numerous in the inner stromal layers, toward the choriocapillaris, 4 2 whereas melanin pigment is more densely concentrated in the outer layers (suprachoroid).16 ,42 The irregular distribution of vessels within the sampling area results in a light reflection from the stroma that is the summation of many components associated with different path lengths through blood and melanin. Although mathematical treatments for light propagation through media with heterogeneous distribution of path lengths have been proposed, 56 their application to the choroid is at present limited by lack of precise information on the content and distribution of both vessels and melanocytes. Instead we have used the simple phenomenological model of Kubelka-Munk.51 This theory assumes that tissue inhomogeneities are small compared to layer thickness and that the incident radiation is diffuse. Although these conditions are not rigorously met, the theory nevertheless offers a means for simple quantitative treatment of light propagation in the choroidal stroma. The introduction of a single unspecified source of light loss in the choroid, in the form of D, is a weakness of model III. However, analysis of D, behavior allows one to draw some conclusions about the model's short- comings. The parameter Dxranges in absolute magnitude between 0 and 0.36 D.U. (Table IV), and is on average larger for the nasal fundus than for the macular sites. The parameter DXtends to increase with Dme,,500 nasally, decreases with Dne,5oo at the fovea, and did not correlate significantly with any of the other three fitted parameters. The effect of the light loss, Dx, is best analyzed, not in absolute terms, but in relation to the contributions of the other absorbers and elements of the model. For the fitted parameter of each spectrum, we calculated the ratio Px of the predicted reflectance with D, = 0 and the predicted reflectance with the actual fitted values of D,. The results indicated that DX has a very low influence in blue and green light (PX< 1.02 at all sites and all spectra), which means that DX is always negligible compared to the absorption by blood and melanin for X < 580 nm. However, in red light, the relative contribution of DX increases rapidly to reach a maximum in the 640-805nm range. The average Px at 675 nm was 1.42 + 0.24, 1.24 ± 0.20, and 1.31 + 0.27 for the nasal fundus, At each of the perifovea, and fovea, respectively. three sites the ratio Px showed a strong tendency, significant at some wavelengths in the 590-805-nm range, to decrease with increasing melanin Dme 500(r = -0.64, -0.60, and -0.61 for the three sites, p - 0.05). This means that the light loss characterized by D, is relatively highest at low pigmentation. A possible explanation for this finding is that light incident in the sampling area diffuses out of the sampling volume by multiple scattering in the choroidal stroma. Such light loss, which is not accounted for in the KubelkaMunk theory, would be more marked in lightly pig- mented fundi. This effect is easily demonstrated by the halo observed around a focally illuminated area on a light fundus. The larger loss observed for the nasal fundus might, in addition to a lower pigmentation, be explained by the fact that a larger sampling area was used at that site (large perimeter, large loss). Another nonconflicting explanation for D. might be related to the shape of the effective absorption spectrum of melanin in the stroma. We have seen in model II (Sec. V.A.3) that a lowering of the fitted scleral reflectance rSc effectively decreased the slope of the absorption spectrum. An increase in D. has the same effect, since the slope is then determined by (Dne, 50oK'me,x + D.) instead of by Dne,500K'mex [Eq. (11)]. At the highest choroidal melanin concentra- tion, no more than 25% of the choroidal volume is occupied by melanin (Sec. V.B.3). Thus, a consider- able amount of light can propagate through the choroid without absorption by melanin, resulting in reflected light contributions that have no choroidal melanin signature. Together with contributions that were absorbed by choroidal melanin, the resulting reflected light would contain a flattened melanin signature.5 7 Thus we expect flattening of the choroidal melanin spectrum, and D, may represent an attempt to correct for the heterogeneity of the choroidal melanin distribution. A final restriction of model III is the use of constant parameters to describe biological entities. In particuwas assumed constant at lar, the RPE density Drpe,500 each site. The individual variability in RPE density was therefore ignored and some of the variability de- tected in Dne,500results in fact from individual variations in RPE melanin. The inability to differentiate between RPE and choroidal melanin is a severe limitation of reflectometry. This is of particular importance in photocoagulation dosimetry19 since the RPE is the major source of heat (largest absorption per unit volume) in fundus photocoagulation. Furthermore, ocular media density Dom,420 was assumed constant for all young subjects in this study. Determination of the individual ocular media density by a psychophysical method 2 7 would clearly improve the modeling, especially if older subjects are to be investigated. VI. Summary and Conclusions The magnitude and shape of the reflectance spectra from the human ocular fundus are critically affected by the amount of melanin in the choroidal stroma. In red light and at low degree of pigmentation, most of the 15 March 1989 / Vol. 28, No. 6 / APPLIEDOPTICS 1075 light originates from the highly vascularized choroid, causing the spectra to reveal pronounced absorption bands of blood. As the degree of pigmentation in- creases, the spectral signature of melanin gradually dominates that of blood, and the contribution of the deeper layer decreases. In green and blue light, the contribution of the choroidal reflections is small compared with that of reflections originating in the retina. This results in a weakening of the spectral signature of blood and in difficulties in quantifying the amount of blood in green light. In red light, quantification of the amount of blood is also complicated by the confound- ing influence of the spectral signature of melanin. The decrease in the apparent amount of blood with increasing degree of fundus pigmentation is a major problem in interpreting the reflectance spectra. We have shown, using an argument involving the spectral signature of the choriocapillaris, that one or several retinal layers must be the origin of substantial reflec- tions. The exact anatomical layer (or layers) responsible for those reflections has not been identified, with the exception of the inner limiting membrane. The retinal reflections are partially responsible for the flattening of the spectral signature of blood with increasing fundus pigmentation. The reflectance spectra also demonstrate the influence of absorption by the macular pigment in blue light and the effect of agerelated changes on absorption by the ocular media. To gain an understanding of the interrelated contribution of the absorption spectra of blood and melanin to the fundus reflectance spectra, one must attempt to model the various constituents of the fundus layers. We first assessed the fundus reflectance model proposed by van Norren and Tiemeijer,10 model II, and found that it adequately fitted the experimental spectra. However, model II suffers from the fact that some model parameters correlate significantly with each other, in ways not consistent with our expectations, based on anatomical evidence. We modified model II by including a light scattering component in the choroid and by introducing several constant parameters corresponding to specific anatomical layers. This model, model III, which has one fewer adjustable parameter than model II, uses a uniform absorbing-scattering layer as an oversimplified representation of the choroidal stroma. Curve fitting of this model to the experimental reflectance spectra produced better regressions to the data and demonstrated an enhanced sensitivity at detecting differences in the amount of choroidal melanin. The interdependence of the different model parameters was markedly reduced, particularly with regard to the relationship between the amount of blood and melanin. The inclusion in the model of an unspecified source of light loss has helped in interpreting the shortcomings of the model and may point the way for improved quantitative modeling of the fundus layers. The authors acknowledge K. A. Fitch for expert technical assistance throughout this project. Special thanks are due to 0. Pomerantzeff, R. Webb, and A. Garsd for numerous discussions and suggestions. 1076 APPLIEDOPTICS / Vol. 28, No. 6 / 15 March 1989 This work was supported in part by grant EY02094 from the National Eye Institute, National Institutes of Health, Bethesda, MD, and by generous support from the Walters Family Foundation, Manhasset, NY. References 1. J. J. Vos, A. A. Munnik, and J. Boogaard, "Absolute Spectral Reflectance (1965). of the Fundus Oculi," J. Opt. Soc. Am. 55, 573 2. R. A. Weale, "Polarized Light and the Human Fundus Oculi," J. Physiol. 186,175 (1966). 3. R. Rohler, U. Miller, and M. Aberl, "Zur Messung der Modulationsiibertragungsfunktion des Lebenden Menschlichen Auges im Reflektierten Licht," Vision Res. 9, 407 (1969). 4. M. Millodot, "Reflection from the Fundus of the Eye and Its Relevance to Retinoscopy," Atti Fond. Giorgio Ronchi 27, 31 (1972). 5. W. N. Charman and J. A. M. Jennings, "Objective Measure- ments of the Longitudinal Chromatic Aberration of the Human Eye," Vision Res. 16, 999 (1976). 6. R. W. Flower, D. S. McLeod, and S. M. Pitts, "Reflection of Light by Small Areas of the Ocular Fundus," Invest. Ophthalmol. Vis. Sci. 16, 981 (1977). 7. D. O'Leary and M. Millodot, "The Discrepancy Between Retinoscopic and Subjective Refraction: Effect of Light Polarization," Am. J. Optom. Physiol. Opt. 55, 553 (1978). 8. W. N. Charman, "Reflection of Plane-Polarized Light by the Retina," Br. J. Physiol. Opt. 34, 34 (1980). 9. J.-M. Gorrand, R. Alfieri, and J.-Y. Boire, "Diffusion of the Retinal Layers of the Living Human Eye," Vision Res. 24, 1097 (1984). 10. D. van Norren and L. F. Tiemeijer, "Spectral Reflectance of the Human Eye," Vision Res. 26, 313 (1986). 11. D. van Norren and J. van der Kraats, "A Continuously Recording Retinal Densitometer," Vision Res. 21, 897 (1981). 12. D. J. Faulkner and C. M. Kemp, "Human Rhodopsin Measurement Using a T.V.-Based Imaging Fundus Reflectometer," Vision Res. 24, 221 (1984). 13. P. E. Kilbride, J. S. Read, G. A. Fishman, and M. Fishman, "Determination of Human Cone Pigment Density Difference Spectra in Spatially Resolved Regions of the Fovea," Vision Res. 12, 1341 (1983). 14. S. Trokel, "Quantitative Studies of Choroidal Blood Flow by Reflective Densitometry," Invest. Ophthalmol. 4,1129 (1965). 15. J. Gloster, "Fundus Oximetry," Exp. Eye Res. 6, 187 (1967). 16. N. H. Bakker, "Fundus Reflectometry, an Experimental Study," Doc. Ophthalmol. 38, 271 (1974). 17. J. Gloster,"Fundus Reflectometry in the Study of the Choroidal Circulation," Int. Ophthalmol. 6,109 (1983). 18. F. Delori, "Noninvasive Technique for Oximetry of Blood in Retinal Vessels," Appl. Opt. 27, 1113 (1988). 19. K. P. Pflibsen, F. C. Delori, 0. Pomerantzeff, and M. M. Pankra- tov, "Fundus Reflectometry for Photocoagulation Dosimetry," Appl. Opt. 28,1084 (1989). 20. W. Hunold and P. Malessa, "Spectrophotometric Determination of Melanin Pigmentation of the Human Ocular Fundus in vivo," Ophthalmic Res. 6, 355 (1974). 21. G. S. Brindley and E. N. Willmer, "The Reflection of Light from the Macular and Peripheral Fundus Oculi in Man," J. Physiol. (London) 116, 350 (1952). 22. K. R. Alexander, P. E. Kilbride, G. A. Fishman, and M. Fishman, "Macular Pigment and Reduced Foveal Short-Wavelength Sensitivity in Retinitis Pigmentosa," Vision Res. 27, 1077 (1987). 23. T. Behrendt and T. D. Duane, "Investigation of Fundus Oculi with Spectral Reflectance Photography. I: Depth and Integrity of Fundal Structures," Arch. Ophthalmol. 75, 375 (1966). 40. D. J. Coleman and F. L. Lizzi, "In vivo Choroidal Thickness Measurement," Am. J. Ophthalmol. 88, 369 (1979). 41. S. Tane, J. Kohno, J. Horikoshi, K. Kondo, K. Ohashi, A. Ko- 24. F. C. Delori, E. S. Gragoudas, R. Francisco, and R. C. Pruett, "Monochromatic Ophthalmoscopy and Fundus Photography," Arch. Ophthalmol. 95, 861 (1977). 25. American National Standards Institute, "Safe Use of Lasers," Z136.1 (ANSI, New York, 1976). 26. F. Delori, J. S. Parker, and M. A. Mainster, "Light Levels in Fundus Photography and Fluorescein Angiography," Vision Res. 20, 1099 (1980). 27. D. van Norren and J. J. Vos, "Spectral Transmission of the Human Ocular Media," Vision Res. 14,1237 (1974). 28. F. C. Delori and K. P. Plibsen, "Reflectance Properties of the Optic Disc," Noninvasive Assessment of the Visual System. OSA Technical Digest, in press. matsu, and T. Kakehashi, "The Study on the MicroscopicBiometry of the Thickness of the Human Retina, Choroid and Sclera by Ultrasound," Acta Soc. Ophthalmol. Jpn. 88,1412 (1984). 42. M. J. Hogan, J. A. Alvarado, and J. E. Weddell, Histology of the Human Eye (Saunders, Philadelphia, 1971). 43. A. Bill, G. Sperber, and K. Ujiie, "Physiology of the Choroidal Vascular Bed," Int. Ophthalmol. 6, 101 (1983). 44. D. M. Snodderly, P. K. Brown, F. C. Delori, and J. D. Auran, "The Macular Pigment. I. Absorbance Spectra, Localization, and Discrimination from Other Yellow Pigments in Primate Retinas," Invest. Ophthalmol. Vis. Sci. 25, 660 (1984). 45. E. A. Boetner and J. R. Wolter, "Transmission of the Ocular Media," Invest. Ophthalmol. 1, 776 (1962). 29. P. R. Bevington, Data Reduction and Error Analysis for the Physical Sciences (McGraw-Hill, New York, 1969), p. 235. 30. Determination of pigment concentrations requires a tight fit 46. J. Walraven, "Spatial Characteristics of Chromatic Induction: the Segregation of Lateral Effects from Straylight Artefacts," both in red light (melanin) and in green light (hemoglobin). Because of the large differences in reflectance in those two spectral bands (Fig. 2), such fit could be achieved by minimizing the relative errors or by using a weight WA = R -2 in Eq. (2). However, a lesser weight might be desired for short wavelengths to minimize the effect of inaccuracies caused by ocular media light scattering, low signal-to-noise ratio, etc. We therefore selected Wx = Robs, as a compromise. 31. J. Neter, W. Wasserman, and M. H. Kutner, Applied Linear Statistical Models (Richard D. Irwin, Inc., Homewood, IL, 1985), p. 580. 32. W. A. Rushton and G. H. Henri, "Bleaching and Regeneration of Cone Pigments in Man," Vision Res. 8, 617 (1968). 33. V. P. Gabel, R. Birngruber, and F. Hillekamp, "Visible and Near Vision Res. 13,1739 (1973). 47. R. S. Smith and M. N. Stein, "Ocular Hazards of Transscleral Laser Radiation. I. Spectral Reflection and Transmission of the Sclera, Choroid and Retina," Am. J. Ophthalmol. 66, 21 (1968). 48. M. Alpern, S. Thompson, and M. S. Lee, "Spectral Transmittance of Visible Light by the Living Human Eye," J. Opt. Soc. Am. 55, 723 (1965). 49. M. T. Mori, R. C. Zeimer, and M. F. Goldberg, "Noninvasive Measurement of Retinal Thickness: a Potential Diagnostic Tool for Macular Edema and Atrophy," Invest. Ophthalmol. Vis. Sci. 29 (ARVO Suppl), 339 (1988). 50. R. C. Zeimer, U. Illinois Eye Research Institute; personal com- Infrared Light Absorption in Pigment Epithelium and Choroid," in Twenty-Third Concilium Ophthalmologicum, Kyoto, 34. 35. 36. 37. munication (June 1988). 51. G. Kortum, Reflectance Spectroscopy (Springer-Verlag, New York, 1969), p. 116. 52. R. L. Longini and R. Zdrojkowski, "A Note on the Theory of K. Shimizu, Ed. (Excerpta Medica, Amsterdam, 1978). I. A. Menon, S. Persad, H. F. Haberman, C. J. Kurian, and P. K. Basu, "A Qualitative Study of the Melanins from Blue and Brown Human Eyes," Exp. Eye Res. 34, 531 (1982). W. J. Geeraets, R. C. Williams, G. Chan, W. T. Ham Jr., D. Guerry III, and F. H. Schmidt, "The Relative Absorption of Thermal Energy in Retina and Choroid," Invest. Ophthalmol. 1, 340 (1962). J. J. Weiter, F. C. Delori, G. L. Wing, and K. A. Fitch, "Retinal Pigment Epithelium Lipofuscin and Melanin and Choroidal Melanin in Human Eyes," Invest. Ophthalmol. Vis. Sci. 27,145 (1986). 36 The in vitro measurements of Weiter et al. were made with polychromatic light in the 500-600-nm spectral range. Using the melanin absorption spectrum of Fig. 4 (solid curve), we calculated that the absorption coefficient at 500 nm should be Backscattering of Light by Living Tissue," IEEE Trans. Biomed. Eng. BME-15, 4 (1968). 53. R. R. Anderson and J. A. Parrish, "The Optics of Human Skin," J. Invest. Dermatol. 77, 13 (1981). 54. S. Takatani and M. D. Graham, "Theoretical Analysis of Diffuse Reflectance from a Two-Layer Tissue Model," IEEE Trans. Biomed. Eng. BME-26, 656 (1979). 55. R. A. Bone and J. M. B. Sparrock, "Comparison of Macular Pigment Densities in Human Eyes," Vision Res. 11, 1057 (1971). 56. D. W. Lubbers and R. Wodick, "Absolute Reflection Photometry Applied to the Measurement of Capillary Oxyhaemoglobin Saturation of the Skin in Man," Oxygen Meas. Biol. Med. 1, 85 (1974). 57. L. N. M. Duysens, "The Flattening of the Absorption Spectrum of Suspensions, as Compared to that of Solutions," Biochim. Biophys. Acta 19,1 (1956). about 1.6 times higher than those reported in Weiter's study. 38. W. Lerche, "Die Kapillarisierung der Menschlichen Retina," in Eye Structure, II Symposium, J. W. Rohen, Ed. (SchattauerVerlag, Stuttgart, 1965). 39. 0. W. van Assendelft, Spectroscopy of Hemoglobin Derivatives (C. C. Thomas, Springfield, IL, 1970). 0 15 March 1989 / Vol. 28, No. 6 / APPLIEDOPTICS 1077