Cr-ACQUISITION AND REACTIVE OXYGEN SPECIES ROLE IN

Volume: I: Issue-2: Aug-Oct -2010 ISSN 0976-4550
Cr-ACQUISITION AND REACTIVE OXYGEN SPECIES ROLE IN GROWTH,
PHOTOSYNTHESIS, PHOTOSYNTHETIC PIGMENTS, AND BIOCHEMICAL
CHANGES IN ESSENTIAL OIL(S) MONOTERPENE OF GERANIUM
(
PELARGONIUM GRAVEOLENS
L.HER.’ EX. AIT.)
A.Misra, A.K. Srivastava, N.K. Srivastava and A. Khan
Central Institute of Medicinal and Aromatic Plants, P.O.CIMAP, Kukrail Picnic Spot
Road Lucknow – 226015, India.
ABSTRACT
: Cr is an essential element required for various metabolic pathways and act as an antioxidant in the various redox-reactions of primary and secondary plant production of – biomolecules. Geranium is an important essential monoterpene oil(s) bearing plant. Culturing the plant at different doses of Cr from 0-1.0 g Cr ml -1 revealed that Cr plays an important role as an in antioxidant promoter, apart from its micronutrient essentiality. 0.25
µ
g Cr ml -1 is the critical concentrations for maximum content of (0.21%) total essential monoterpene oil(s). At concentration below and above 0.25 g Cr ml -1 , the CO
2
assimilation rate, photosynthetic pigments content and ultimately the accumulation of essential monoterpene oil(s) are affected.
The maximum peroxidase and SOD activities were obtained at 0.25 g Cr ml -1 , with the production of biomolecule geraniol. Results revealed an oxido-reducable reaction of Cr in the formation of monoterpene essential oil(s) and possibly for the major constituents Geraniol.
Keywords
: Cr, Photosynthesis, Geranium, Monoterpene oils
International Journal of Applied Biology and Pharmaceutical Technology
Page: 410
Available online at www.ijabpt.com
Misra et al
INTRODUCTION
ISSN 0976-4550
Heavy manuring - N,P,K and excessive vermiculturing as an organic fertigation to increase the crop productivity and sustainability leads, to the major environmental hazardous and limiting factors in crop production . The general response of plants to increase the ion concentration , includes osmotic stress, specific ion toxicity and further the nutrient deficiencies, thus affecting a range of physiological processes involved in cell metabolism( Munns,2002)Further at higher altitudes cultivation . the low pH and high Fe toxicity, apart from heavy metal toxicity due to vermiculturing in plains affects the productivity and biomass of the plants. These cumulative effects of salts complexes and heavy metal in roots , including the other environmental stresses , are known to be mediated atleast partially , by an advocative enhanced generation of Reactive Oxygen Species ( ROS )and free radicals. ROS such as hydrogen peroxide (H
2
O
2
), singlet oxygen ( ‘ O2), super oxide anion radicals (-O2), hydroxyl radicals(-OH) are generated from the autoxidation of lipids and H
2
O
2
production through water-water cycle (Asada 1999, Havaux et al.
2003)Leaf antioxidant systems include the enzymes and antioxidant metabolites of the so called waterwater cycle, wchich functions to scavange superoxide that is produced from the reduction of oxygen by PSI
(Asada,1999). In the water-water cycle, superoxide dismutase(SOD) catalyses the conversion of superoxide to hydrogen peroxide,which is then converted to water via the enzyme ascorbate peroxidase using the ascorbate as a reductant. Formations of these ROS by strong sun shine and UV irradiations arelikely to damage several cellular components such as lipids,proteins, nucleic acid and DNAs through oxidation
(Singh 1989, Sawa 2003). Plants contain a wide variety of chemicals that have potent antioxidants – β carotein, α -tocopherol, ascorbates, Vit.E, Cr, Zn, and Se metals which effects overall metabolism and physiology of the plants through antioxidants activities in scavenging the free-radicals formed in stressescaused by intrinsic ,extrinsic biotic and abiotic factors. Stresses causes a severe imbalence between light absorption and its utilization via photosynthesis . In such conditions , plants emply photoreceptive mechanisms to deal with excess light absorbed by chlrophyll . Apart, from Cr being antioxidant in oxidoreduceable reactions during the uptake of Fe +2 from soils for reactions with aqueous Cr(IV) species , and also with the rate of Cr(IV) reduction by organic matter ( Eary and Rai, 1989,1991). Thus stimulating effects to the additions of small amount of Cr on plant growth and yield have been observed by several researchers(Bonet et al.1991; Pratt, 1966; Adel M Zayed and Terry, 2003; Terry, 1981). During the Fe deficiency the Cr enhances the removal of Fe chlorosis by incrasing the Fe +2 uptake(Terry,1981).No conclusive evidence yet of an essential role of Cr in plant metabolisms but to effect the growth and productivity of plants at low concentration utilization Huffman and Allaway,1973), either by solublization of Fe +3 to Fe +2 active Fe for plant uptake or in in an antioxidant activities for defence mechanismsin controlling ROS.Further the stimulation of plant growth in response to Cr(IV) for molybdate
( Warington,1946).The intraction between Cr(III) and Fe, P and Mn was extensively studied in Fe-deficient plants to reduce the Fe chlorosis( Bonet et al.1991). The positive effects of Cr in plant growth and physiology is to influence thelevels of cytokinins , interactions with nucleic acids, competitive inhibition on the binding of spermine to DNA , or Cr induced increase in the levels of the polyamine- spermine(Poschenrieder et al.1991), wchich in turns effects the ultrastuctures of chloroplasts. Therfore the acquisition of Cr on growth and physiology of Geranium with antioxidant activities will be studied in detail .
International Journal of Applied Biology and Pharmaceutical Technology
Page: 411
Available online at www.ijabpt.com
Misra et al ISSN 0976-4550
Geranium, Pelargonium graveolens L. `Her, ex Ait. Syn., P. roseum Willd of family Geraniaceae is the only source of one of the most important essential monoterpene oil(s) called the oil of geranium. It is commonly known as rose geranium (Anonymous, 1966, 1975, 1982 and Erickson, 1976). It is distinctly different from the horticultural Geranium, which are basically the ornamental and have no commercial usage in perfumery industries (Douglas, 1969). P. graveolens widely grown varieties are Algerian/Funition,
Kelkar/Egyption and Bourbon/Reunion (Rajeshwar Rao and Bhatacharya 1992) is popularly cultivated in
India. Steam distillation of the shoot biomass of Geranium yields the geraniol and citronellol rich monoterpene oil(s), extensively used for perfuming soaps, and cosmetics to which it imparts a pronounced and lasting rose odour, for high grade perfumes. It is largely used in flavoring tobacco products, tooth pastes, ointments and other pharmaceutical preparations (Ranade, 1988).
Cr is an essential mirconutrient that acts either as a metal component of various enzymes or as a functional, structural or regulatory cofactor, and is thus associated with saccharide metabolism, photosynthesis, and protein synthesis (Marschner, 1986). Zn-deficiency reduces plant growth and inhibits photosynthesis in a wide variety of plants including forest tree (Dell, and Wilson 1985), fiber crop (Ohki, 1976), rice (Ajay and
Rathore, 1995) and spinach (Randall and Bouma, 1973). Cr retards the activity of carbon metabolism enzymes such as carbonic anhydrase, (Ohki, 1976, 1978), ribulose 1,5 bisphosphate carboxylase/oxygenase, and fructose – 1,6- bisphosphate (Marshner 1986). Zn, Se and Cr all play an important role in an antioxidation to scavenging the free radicals. Cr role as a antioxidant is to stimulate the removal of free-radicals (Chakamak and Engels, 1999).
Essential oil biosynthesis in Geranium is strongly influenced by Cr-acquisition and the stresses caused by
Cr on nutrition and growth. Cr is involved in carbon assimilation and saccharide accumulation, free radical removal, in antioxidant enzymes and role in carbon utilized in terpene biosynthesis and the overall growth of the plants. Furthermore, the role of Cr in influencing essential monoterpene oil(s) accumulation seems particularly important. The requirement of Zn antioxidant for Japanese mint and Pelargonium and its limitations imposed on photosynthetic carbon metabolism and translocation in relation to essential oil accumulation in mint have been documented by Misra and Sharma (1991) and Misra et al. (2004), respectively. While Cr as an antioxidants enzymes for free radical quenching in Geranium have not fully been documented and not much work has been done so far.
In the present paper we report on the role of – Cr, as a stimulant of free-radicals quenching through Cr affected antioxidants enzyme activity, in Geranium subjected to Cr stress. Simultaneously, photosynthetic efficiency in terms of changes in CO
2
exchange rate, photosynthetic pigments, leaf fresh mass, dry mass, leaf area, Cr content in whole plant shoot biomass and oil yield were also determined.
Materials and Methods :
Plant : Plant tips (5-6 inches) with 3-4 leaves of Geranium ( Pelargonium graveolens ) of Bourbon genotype were obtained from the farm nursery of the CIMAP, Lucknow, India. Uniform cuttings were initially planted in 10000 cm 3 earthen pots filled with purified silica sand (Agarwala and Sharma, 1961), for the development of roots. After 15 days, rooted cuttings were transferred to 2500 cm 3 pots. The salts used in nutrient solution culture were purified for Zn (Hewitt, 1952). Hoagland and Arnon’s (1959) nutrient solution was used in the experiment except Fe which was supplied as Fe-EDTA. Three pots each of Zn treatments ranging from 0.0 to 1.0 g Zn ml -1 ambient temperature (30+5 o
were maintained in controlled glass house condition at
C) and irradiance (between 800-1000 mol m¯2 s¯1). The nutrient solution in each treatment was added at alternate days. With onset of deficiency and toxicity (after 20 days) growth observation and detailed physiological and biochemical data with growth attributes were performed.
International Journal of Applied Biology and Pharmaceutical Technology
Page: 412
Available online at www.ijabpt.com
Misra et al ISSN 0976-4550
CO
2
exchange rate (P
N
) : CO
2
exchange rate was measured using a computerized portable photosynthesis system (Srivastava and Misra, 1991) (Model LiCOR 6000, LiCOR, USA).
Chlorophyll (Chl) content : A known mass of leaf tissue (3 rd leaf) was extracted with 80% acetone and observance was recorded on spectrophotometer (Pye Unicham PU8610, USA) for determination of Chl a and b according to Arnon (1949)., and Carotenoids were calculated as described by Deming-Adams(1992).
Growth attributes and Zn analysis : Leaf fresh and shoot dry mass and area (area meter LICOR Li-3000) were recorded. For tissue elemental analysis 1.g. dried leaf samples were digested with IN HCl at 60 o C for
24 h. Aliquot samples of the clear digest were diluted with water (10 cm 3 ) and analyzed for Zn by atomic absorption spectrophotometer (Pye Unicam SP 2800) (Misra and Sharma 1991). Antioxidant reactive peroxidase enzyme activity was estimated as described earlier (Shanon et al ., 1960). Using 2 g of fresh chopped leaves at position 3rd, were homogenized with 5 ml of 0.1 M phosphate buffer (pH 6.8). Each treatment was replicated 3 times and assayed on SDS page electrophoresis. Superoxide Dimutase (SOD) activity were assayed by the method of Henary et.al (1976).
Estimation of essential monoterpene oil(s) : Geranium oil estimation was done by steam distillation of
100 g freshly plucked leaves in a clevenger’s apparatus (Clevenger, 1928). The oil constituents mainly
Geraniol, citronellol and other associated oil contents were determined by Gas liquid chromatography
(Perkin –Elemer model 3920 B). The stainless steel column was packed with 10% carbowax (20 mesh) on chromosorb WNAW. Injector and detector temperature was maintained at 200 cm S o C. The flow of H
-1 data processing for area % was done on a Hewllet Packard integrator model HP-33%.
2
was 0.47
Statistical Analysis : The results were statistically analyzed for the Least Significant Differences (LSD) using the layout of a complete randomized design (CRD). Further the results were analyzed for the correlation coefficient to determine the relationship among the characters studied, using the relationship Y= a+b x.
Results and Discussion
The fresh and dry biomass increased with increase in the level of Zn. (Table 1). Maximum fresh (282.5 g/plant), dry biomass (19.36 µg/plant) and leaf area (40.3cm2) was observed at the 0.25 µg Zn ml
Growth attributes such as, plant height (64.1 cm) was maximum at 0.50 µg Zn ml
µg Zn ml -1
-1
-1 supply.
supply. Zn levels of 1.0
were toxic to overall growth parameters. The total chlorophyll content increased upto 0.25
µgZn/ml and then decreased; where as same trend was observed for Chl.a and Chl.b. While Carotenoids were increased from deficient levels of Cr supply to 0.25 µg Zn ml -1 supply. The maximum photosynthetic efficiency in terms of CO2 exchange rate of 0.82 µgCO2/m2/s was found at 0.25 µg Zn ml saccharide formation was also found to be highest at 0.25 µg Zn ml inhibits P
N
-1
-1 supply. The
supply. Zn deficiency and Zn toxicity
in cotton (Ohki, 1976), peppermint (Srivastava et al ., 1997), Soybean (Ohki, 1978) and sweet mint (Misra et al 2004). A decrease in Chl. photosynthetic pigment content represents a decline in photochemical capacity of leaf at deficient Zn supply. (Ohki, 1976)
International Journal of Applied Biology and Pharmaceutical Technology
Page: 413
Available online at www.ijabpt.com
Misra et al ISSN 0976-4550
Peroxidase activity and peroxidase isoenzymes maximum bands were observed at 0.25 µg Zn ml -1 cultured plants. The Zn deficient and toxic cultured plants revealed lesser peroxidase activity with lesser peroxidase isoenzymes band profiles. SOD activity showed as Nitro Blue Tetrazollium (NBT) reduction.The maximum SOD (0.248 units/mg.fr.wt.) was obtained at 0.25 µg Zn ml -1 cultured plants. In Japanese mint similar report were observed for Mn nutrition (Misra 1996). The maximum monoterpene oil(s) was found at 0.25 g Zn ml -1 treatments. However with respect to oil composition, contents of citronellol. Geraniol , linalool and nerol varied at different Zn treatments (Table 2).As a result of differential Zn supply the content of other micronutrient as Fe, Mn, Zn, and Cu were affected and showed lesser Fe, Mn, Zn and Cu concentrations in shoot tissue of Geranium (Table 3). The maximum (Zn 164 µg g -1 ), Cu (12 µg g -1 ) Mn
(98 µg g -1 and Fe (537 µg g -1 ) contents were observed at 0.25 µg Zn ml -1 treatments.
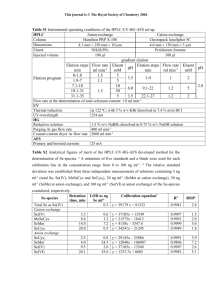
Table 1: Effect of Cr acquisition on growth parameters of Geranium
.
Treatment
0.0 µg Cr ml -1
0.005 µg Cr ml -1
0.05 µg Cr ml
0.10 µg Cr ml
-1
-1
0.25 µg Cr ml -1
0.50 µg Cr ml -1
Plant height (cm)
57.0
58.0
61.0*
62.5**
63.4**
64.1**
No. of branches/ plant
(Nos.)
9
10*
13**
18**
10*
10*
Fresh weight /plant
(g)
218.8
238.6*
224.8
252.1**
282.5**
215.5
1.00 µg Cr ml
LSD at 5%
1%
-1 59
2.5
4.1
8
1.1
3.2
196.2
11.1
16.3
* ** Values are significantly different at 5% and 1% level.
Dry weight/ plant (g)
14.11
16.33*
16.81*
17.37**
19.36**
18.46**
15.85
2.1
3.3
Leaf area
(cm) 2
8.2
12.1*
25.2**
39.1**
40.3**
37.2**
11.2
3.5
6.2
Table 2: Effect of Cr on photosynthetic pigments, photosynthesis (P
N and saccharide formation of Geranium.
), chlorophyll (Chl) contents
Caroten-
Treatment
Chl a mg g -1 f.wt.
Chl b mg g -1 f.wt.
Chl a+b mg g -1 f.wt.
oids mg g f.wt.
-1
P
N
µg(CO2) m -2 s -1
Saccharides µg
(CH
2
O) m -2 s -1
0.0 µg Cr ml
0.10 µg Cr ml
0.25 µg Cr ml
LSD at 5%
1%
-1
0.005 µg Cr ml -1
0.05 µg Cr ml -1
-1
-1
0.50 µg Cr ml -1
1.00 µg Cr ml -1
0.68
0.79*
0.94**
1.35**
1.48**
1.01**
0.82*
0.11
0.15
0.50
0.56
0.61*
0.69**
0.79**
0.40
0.29
0.08
0.12
1.18
1.35**
1.55**
2.04**
2.27**
1.41**
1.11
0.07
0.11
* ** Values are significantly different at 1% and 5% level.
0.086**
0.051**
0.024**
0.016**
0.007**
0.003*
0.002
0.0002
0.0005
0.15
0.19*
0.75**
0.76**
0.82**
0.71**
0.42**
0.03
0.06
0.102
0.129
0.510 **
0.516**
0.558**
0.483**
0.286*
0.05
0.07
International Journal of Applied Biology and Pharmaceutical Technology
Page: 414
Available online at www.ijabpt.com
Misra et al ISSN 0976-4550
Statistical analysis showed a positive significant association between Zn content in leaf and total CO
2 exchange rate (P
N
) (µg =0.924 < p=0..5%) and between P
N
and saccharides (sugars) (µg =0.879 < p=0.05% content .However, Zn content in leaf was negatively correlated with Chl. a/b ratio. The CER showed a positive significant association with leaf fresh mass (µg = 791 < p=0.05%), leaf dry mass (µg
=692 < 0.05%), leaf area and total monoterpene oil(s) (=0.721 < p=0.01). A positive significant correlation was also observed between saccharides and total oil (µg =0.695 < p=0.01%). A quadratic trend was observed for all these characters which were comparable in + Zn then the deficient and much higher
Zn cultured plants.
Table 3: Effect of Cr acquisition on geranium total oil and oil composition.
Treatments % of oil
Citronellol
% of total oil
Geraniol
% of total oil
Linalool
% of total oil
Nerol
% of total oil
0.0 µg Cr ml -1
0.005 µg Cr ml -1
0.15
0.16
0.21
0.27**
0.09
0.09
8.0
10.0**
1.1
1.2**
0.05 µg Cr ml -1
0.10 µg Cr ml -1
0.25 µg Cr ml -1
0.50 µg Cr ml -1
0.17*
0.19
0.21**
0.16
0.29**
0.32**
0.25**
0.18**
0.10**
0.11**
0.07**
0.12**
1.00 µg Cr ml
LSD at 5%
1%
-1 0.15
0.02
0.04
0.17**
0.01
0.02
0.10**
0.005
* ** Values are significantly different at 5% and 1% level.
0.01
9.0**
6.0**
7.0**
8.0**
7.0**
0.04
0.07
1.1
1.4**
1.2**
0.9**
0.7**
0.01
0.02
Table 4: Effect of Cr acquisition on tissue concentrations in shoots of Geranium.
Treatments Fe (µg g -1 ) Mn (µg g -1 ) Zn (µg g -1 ) Cu (µg g -1 )
0.0 µg Cr ml -1
0.005 µg Cr ml -1
98
112
26
37**
12
19*
7
9
0.05 µg Cr ml -1
0.10 µg Cr ml -1
142**
249**
537**
41**
57**
98**
34**
45**
64**
11*
11
12** 0.25 µg Cr ml -1
0.50 µg Cr ml -1
1.00 µg Cr ml -1
LSD at 5%
419**
312**
21
62**
53**
9
41**
36**
7
7
5
3
1% 42 11 9
* ** Values are significantly different at 5% and 1% level
5
The observed maximum changes in CER, photosynthesis pigments content, leaf area, and antioxidant enzymes- peroxidase activities along with other leaf physiological parameters at 0.25 µg Zn ml -1 supply indicate that this level is optimum for best growth. At this Zn supply activity of peroxidase and its isoenzymes ,the highest CER ,chlorophyll content, and saccharides results in maximum flow of photosynthates to the biosynthetic pathways. The main sites of ROS production in the plant cells are the organelles with highly oxidizing metabolic activities or with sustained electron flows: chloroplasts,mitrochondria and peroxisomes.( Garnczarska et al. 2004). In chloroplasts ROS can be generated by the direct transfer of the excitation energy , or by oxygen reduction in the Mehler reaction
( Meloni et al. 2003).In addition , H
2
O
2 oxido-reducible processes( Misra, 2003).
and O
2
- may interact in the presence of certain ions (Co, Al, Cu,
Cd.Zn, Se) and Cr chelates to produce highly reactive OH( Sudhakar et al.2001 ) Antioxidants is thus
International Journal of Applied Biology and Pharmaceutical Technology
Page: 415
Available online at www.ijabpt.com
Misra et al ISSN 0976-4550
In healthy unstreesed conditions to the plants not to damage the cell walls and normal physiological activities persists but during the biotic and abiotic stresses of extrinsic factors leads to the antioxidant and antioxidant oxidizing metabolites .
Further closing of the stomata and stomatol resistance, for utilization of very low concentration of carbon dioxide which enables then to assimilates carbon dioxide even during considerable stomatol closure( El
Bassam, 1998). Thus the study was to examine the stresses of envirnment as well the extrinsic biotic factors to examine simultaneously both ROS of Xanthophyll cycle of carotenoids for dependent energy dissipation and leaf antioxidant system – specially elemental antioxidant chromium(Cr) acquisition in Geranium and its essential monoterpene oil(s) secondary plant products.
The simultaneous reduction in growth, CER content of other micronutrient ,and peroxidase activity in leaves indicate the limited availability of photosynthates to essential metabolism and biosynthesis.
Utilization of metabolites from primary photosynthetic process into secondary metabolites regulates monoterpene productions (Gershenzon and Croteau, 1991). Thus a close relation between photosynthesis, photorespiaration and terpenoid synthesis also exists in essential monoterpene oil(s) bearing plants (Maffei and Codignola 1990). Moreover, the actively growing leaves requires a larger supply of an antioxidants stimulator Zn, in associations with greater supply of photosynthates and since essential oil biosynthesis occurs in these rapidly growing leaves, the initial growth period would require a still greater supply of photosynthates and energy .
Acknowledgement : The authors are thankfull to the Director , CIMAP for the encouragement and facilities provided during the studies.
REFERENCES
Agrawala, S.C., Sharma, C.P.: The standardization of sand and water culture technique for the study of macro and micronutrients (trace) element deficiencies under Indian conditions. - Curr. Sci .40
:424-428,
1961.
Ajay. Rathore, V.S.: Effect of Zn stress in rice (Oryza sativa cv. Manhar) on growth and photosynthetic processes. Photosynthetica. - 31 : 571-584, 1995.
Anonymous,: Wealth of India, Raw Materials, Vol. 7, CSIR, New Delhi, p. 287,1966.
Anonymous, Annual Report. Horticultural Research Station, Kodaikanal, Tamil Nadu, 1975.
Anoonymous.: Monthly Statistics of the Foreign Trade of India Vol. 11, March,1982.
Arnon, D.I.:Copper enzymes in isolated chloroplasts. Polypheol oxidase in Beta vulgaris . Plant Physiol.-
24 : 1-15,1949.
Asada,K.: The water-water cycle in chloroplasts : scavenging of active oxygen and dissipation of excess photons .- Annu Rev Plant Physiol Plant Mol Biol.
50 :601-639,1999.
Bonet, A.,Poschenrieder, C,., Barcelo, J.:Chromium III-iron interaction in Fe-deficient and Fe sufficient bean plants.1.Growth and nutrient content.- J Plant Nutr. 14 :403-414,1991.
Clevenger, J.F.: Apparatus for determination of essential oils. - J. Amer. Pharmac. Assoc. - 17 : 346, 1928.
Dell, B.,Wilson, S.A.: Effect of Zn supply on growth of three species of Eucalyptus seedlings and wheat.-
Plant Soil. 88 : 377-384,1985.
Douglas, J.S.: Essential oil crops and their uses.- World Crops . 21 : 49-54,1969.
Erickson, R.E.: Industrial importance of monoterpenes and essential oils.- Lyodia . 29 : 8-19,1976.
Gershenzon, J.,Croteau, R.: Regulation of monoterpene biosynthesis in higher Plants. In: Towers, G.H.N.,
Safford, H.A. (ed.): Biochemistry of the mevalonoic Acid pathway to terpenoids. pp. 99-159. Plemem
Press, New York, 1991.
International Journal of Applied Biology and Pharmaceutical Technology
Page: 416
Available online at www.ijabpt.com
Misra et al ISSN 0976-4550
Henary, L., Halliwell, B., Hall, D.:The Superoxide dismutase activity of various photosynthetic organisms measured by a new rapid assay techniques.- FEBS.
66 : 303.
Hewitt, E.J.: Sand and water culture methods used in the study of plant nutrition,-Commonwealth Bureau
Bot. Plantation Crops Tech. Commun . 22 : 405-439, 1952.
Hoagland, D.R.,Arnon, D.I.: The water culture method for growing plants without soil.- Calif. Agric. Exp.
Statio .Circ
. 347 : p. 32,1952.
Huffman Jr, E W., Allaway W H .: Chromium in plants: distribution in tissue, organelles and extracts , and availability of bean leaf Cr to animals. – J Agric Food Chem.
21 ; 982-986, 1973.
Maffei, M., Codignola, A.: Photosynthesis and herbicide effect on terpene production in peppermint
( Mentha piperita L.).- J. Essen. Oil Res . 2: 275-286, 1990.
Marschner, H.: Function of mineral nutrients. Micronutrients In: Mineral Nutritional of higher plants. Pp.
269-300, Academic Press, New York, 1986.
Misra, A.: Genotypic variation of Manganese toxicity and tolerance of Japanese mint.- J. Herbs, Spices and
Medicinal Plants . 4 : 3-13, 1996.
Misra, A., Sharma S.: Zn concentration for essential oil yield and menthol concentration of Japanese mint.-
Fertilizer Res . 29 : 261-265,1991.
Misra,A., Srivastava,N.K., Sharma,S.: Role of an antioxidant on net photosynthetic rate,carbon partitioning and oil accumulation in sweet mint.-Agrotropica.15:69-74,2003.
Munns, R.:Comparative physiology of salt and water stress.-Plant Cell. Environ.
25 :239-250,2002.
Ohki, K.: Effect of Zn nutrition on photosynthesis and carbonic anhydrase activity in cotton. - Physiol.
Plant . 38 : 300-304, 1976.
Ohki, K.: Zinc concentration of soybean as related to growth, photosynthesis and carbonic anhydrase activity.- Crop. Sci. 18 : 79-82, 1978.
Poschenrieder, D., Vazquez, M D ., Bonet, A.,Barcelo, J.:Chromium III-iron interaction in iron sufficient and iron deficient bean plants.2. Ultrastructural aspects. J.Plant Nutr.
14 :315-428,1991.
Pratt, P F .: Chromium.
in Diagnostic Criteria for plants and Soils.Ed. H.D. Chapman. Ch. 9, pp. 136-141,
University of California,Riverside, 1966.
Rajeshwar Rao B.R., Bhatacharya, A.K. : History and botanical nomenclature of rose scented geranium cultivars grown in India.- Indian Perfumer . 36 : 155-161, 1992..
Ranade, G.S.: Chemistry of Geranium of Geranium oil.-Indian Perfumer . 32 : 61-68, 1988.
Randall, P.J., Bouma, D.: Zinc deficiency, carbonic anhydrase and photosynthesis in leaves of Spinach.-
Plant Physiol. 52 : 229-232,1973.
Sawa, T., Akaike, T.,Maeda, H.: Tyrosinenitration by peroxinitite formed from nitric oxide and superoxide generated by Xanthine oxidase.- J.Biol.Chem.
275 :32467-32474,2000.
Sharon, L.M., Kay, E., Lew, J.Y. : Peroxidase isoenzymes from horse raddish roots 1-1 Isolation and
Physical properties.- J. Biol. Chem. 241 : 2166-2172,1966.
Singh, A.: Chemical and biochemical aspects of activated oxygen: singlet oxygen, superoxide anion and related species. In CRC Handbook of free radicals and antioxidants in Biomedicine . Mequel J.
Quintanilha, A T., Weber, H. eds. CRC press, inc., Boca Raton, FL, USA. Vol.1 pp. 17-28, 1989.
Srivastava, N.K., Misra, A., Sharma, S.: Effect of Zn deficiency on net photosynthetic rate, 14 C partitioning, and oil accumulation in leaves of peppermint.- Photosynthetica . 33 : 71-79, 1997.
Sudhakar, C., Lashmi, A., Giridarakumar, S.:Changes in the antioxidant enzymes efficacy in two high yielding genotypes of mulberry( Morus alba L.) under NaCl salinity.- Plant Sci.
161 :613-619,2001.
Terry, N.: An analysis of the growth responses in Beta vulgaris L. to phototoxic trace elements . II.
Chromium. Final report to the Kearney Foundation of Soil Science. July, 1975 - June,1980.
Warington, K.: Molybdenum as a factor in the nutrition of lettuce.- Ann Appl Biol 33 : 249-254,1946.
International Journal of Applied Biology and Pharmaceutical Technology
Page: 417
Available online at www.ijabpt.com