me 211 hw #4 solution
advertisement

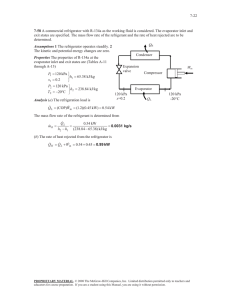
CANKAYA UNIVERSITY FACULTY OF ENGINEERING AND ARCHITECTURE MECHANICAL ENGINEERING DEPARTMENT ME 211 THERMODYNAMICS I FALL 2015 HW # 4 SOLUTION 1) Air at 10 0C and 80 kPa enters the diffuser of a jet engine steadily with a velocity of 200 m/s. The inlet area of the diffuser is 0.4 m2. The air leaves the diffuser with a velocity that is very small compared with the inlet velocity. Determine (a) the mass flow rate of the air and (b) the temperature of the air leaving the diffuser. Solution (b) SSSF conditions Conservation of mass me mi e i Single inlet single outlet m2 m1 m First Law of Thermodynamics 1 1 QCV WCV me (h e Ve2 gz e ) mi (h i Vi2 gz i ) 2 2 e i QCV WCV 0, V2 0, PE 0 1 0 me h e mi (h i Vi2 ) 2 e i Single inlet single outlet 1 m(h1 V12 ) mh 2 2 1 h 2 (h1 V12 ) 2 At the inlet T1 10 0C p1 80 kPa 2) Air at 100 kPa and 280 K is compressed steadily to 600 kPa and 400 K. The mass flow rate of the air is 0.02 kg/s, and a heat loss of 16 kJ/kg occurs during the process. Assuming the changes in kinetic and potential energies are negligible, determine the necessary power input to the compressor. Solution (b) SSSF conditions Conservation of mass me mi e i Single inlet single outlet m2 m1 m First Law of Thermodynamics 1 1 QCV WCV me (h e Ve2 gz e ) mi (h i Vi2 gz i ) 2 2 e i KE 0, PE 0 QCV WCV me h e mi h i e i Single inlet single outlet QCV WCV m(h 2 h1 ) Qout ( WC ) m(h 2 h1 ) Qout mq out WC mq out m(h 2 h1 ) p1 100 kPa h1 280.13kJ / kg T1 280 K p1 600 kPa h 2 400.98 kJ / kg T1 400 K 3) The power output of an adiabatic steam turbine is 5 MW, and the inlet and the exit conditions of the steam are as indicated in Figure given below (a) Compare the magnitudes of h, KE, PE (b) Determine the work done per unit mass of the steam flowing through the turbine. (c) Calculate the mass flow rate of the steam. Solution SSSF At the inlet: p1 2 MPa 20 bar Tsat 212.4 0C T1 Tsat At the outlet: Let us compute h, KE, PE b) Conservation of mass me mi e i Single inlet single outlet m2 m1 m First Law of Thermodynamics 1 1 QCV WCV me (h e Ve2 gz e ) mi (h i Vi2 gz i ) 2 2 e i Single inlet single outlet 1 1 QCV WCV me (h e Ve2 gz e ) m i (h i Vi2 gz i ) 2 2 1 QCV WCV m (h e h i ) (Ve2 Vi2 ) g(z e z i ) 2 1 Wt m (h e h i ) (Ve2 Vi2 ) g(z e z i ) 2 Neglecting heat loss, 4) QCV 0 1 Wt m (h 2 h1 ) (V22 V12 ) g(z 2 z1 ) 2 or W 1 w t (h 2 h1 ) (V22 V12 ) g(z 2 z1 ) kJ / kg m 2 4) Refrigerant-134a enters the capillary tube of a refrigerator as saturated liquid at 0.8 MPa and is throttled to a pressure of 0.12 MPa. Determine the quality of the refrigerant at the final state and the temperature drop during this process. Solution 5) Refrigerant-134a is to be cooled by water in a condenser. The refrigerant enters the condenser with a mass flow rate of 6 kg/min at 1 MPa and 70 0C and leaves at 35 0C. The cooling water enters at 300 kPa and 15 0C and leaves at 25 0C. Neglecting any pressure drops, determine (a) the mass flow rate of the cooling water required and (b) the heat transfer rate from the refrigerant to water. SSSF conditions Conservation of mass me mi e i m 2 m 4 m1 m 3 m3 m4 m R m1 m 2 m w m R mass flow rate of R-134a m w =mass flow rate of water\ First Law of Thermodynamics 1 1 QCV WCV me (h e Ve2 gz e ) mi (h i Vi2 gz i ) 2 2 e i KE 0, PE 0,QCV 0, WCV 0 m h m h e e e i i i m4 h 4 m 2 h 2 m1h1 m3h 3 m R (h 4 h 3 ) m W (h1 h 2 ) Now we need to determine the enthalpies at all four states. Water exists as a compressed liquid at both the inlet and the exit since the temperatures at both locations are below the saturation temperature of water at 300 kPa. At inlet 1: T1 15 0C p1 300 kPa p1 300 kPa Tsat 133.6 0C T1 Tsat compressed liquid At outlet 2: T1 25 0C p1 300 kPa p2 300 kPa Tsat 133.6 0C T2 Tsat compressed liquid State-3 p3 1MPa 10 bar Tsat 39.39 0C T1 Tsat State-4 p4 1MPa 10 bar Tsat 39.39 0C T4 Tsat Compressed liquid So the heat lost by hot fluid is equal to heat gained by cold fluid See CV on next page QW QR QW m w (h 2 h1 ) 6) An insulated 8-m3 rigid tank contains air at 600 kPa and 400 K. A valve connected to the tank is now opened, and air is allowed to escape until the pressure inside drops to 200 kPa. The air temperature during the process is maintained constant by an electric resistance heater placed in the tank. Determine the electrical energy supplied to air during this process. Solution USUF process Conservation of mass: m2 m1 cv mi me i i Conservation of energy 𝑄𝐶𝑉 + mi hi i = me he e Vi 2 gzi 2 𝑉22 𝑉2 Ve 2 + 𝑔𝑧2 ) − 𝑚1 (𝑢1 + 1 + 𝑔𝑧1 )] + Wcv gze +[𝑚2 (𝑢2 + 2 2 2 𝐶𝑉 Assumptions 1) This is an unsteady process since the conditions within the device are changing during the process, but it can be analyzed as a uniform flow process since the exit conditions remain constant. 2) Kinetic and potential energies are negligible. 3 ) The tank is insulated and thus heat transfer is negligible. 4) Air is an ideal gas with variable specific heats. For this reason, we will use air tables. We take the contents of the tank as the system, which is a control volume since mass crosses the boundary. Noting that the microscopic energies of flowing and non- flowing fluids are represented by enthalpy h and internal energy u, respectively, the mass and energy balances for this uniform-flow system can be expressed as Conservation of mass. No mass is entering. So m2 m1 cv mi me i me m1 m2 cv i Conservation of energy KE 0, PE 0, no mass is leaving since tank is insulated QCV 0, Electrical work is done on the CV We me h e +[𝑚2 (𝑢2 ) − 𝑚1 (𝑢1 )]𝐶𝑉