The International System of Units
advertisement

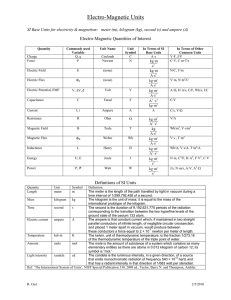
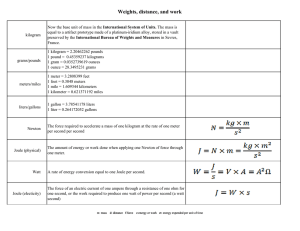
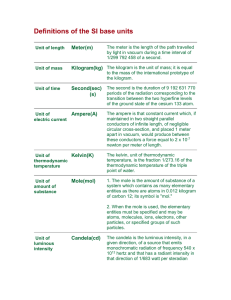
The International System of Units Base Units The International System of Units (Systeme International d'Unites, with the international abbreviation SI), is the modern version of the metric system adopted by the General Conference of Weights and measures in 1960. It has seven independent base units. meter (metre), m: The meter is the length of the path traveled by light in vacuum during a time interval of 1/299792458 of a second. kilogram, kg: The kilogram is the unit of mass; it is equal to the mass of the international prototype of the kilogram. second, s: The second is the duration of 9,192,631,770 periods of the radiation corresponding to the transition between the two hyperfine levels of the ground state of the cesium-133 atom. ampere, A: The ampere is the constant current which, if maintained in two straight parallel conductors of infinite length, of negligible circular cross section, and place 1 meter apart in vacuum, would produce between these conductors a force equal to 2 x 10-7 newton per meter of length. kelvin, K: The kelvin, the unit of thermodynamic temperature, is the fraction 1/273.16 of the thermodynamic temperature of the triple point of water. The unit kelvin and its symbol, K, should be used to express both the thermodynamic temperature and an interval or a difference of temperature. Just say "kelvin", not "degrees kelvin". mole, mol: The mole is the amount of substance of a system which contains as many elementary entities as there are atoms in 0.012 kilogram of carbon-12. When the mole is used, the elementary entities must be specific and may be atoms, molecules, ions, electrons, other particles, or specified groups of such particles. candela. cd: The candela is the luminous intensity, in a given direction, of a source that emits monochromatic radiation of frequency 540 x 1012 hertz and that has a radiant intensity in that direction of 1/683 watts per steradian. From The Physics Quick Reference Guide by E. Richard Cohen. AIP press, 1996. Table 1. Prefixes used in SI. These prefixes are used to indicate multiples or submultiples of the base unit, except that units for mass are formed by applying the prefix to the symbol g: i.e., Mg not kkg and mg not μkg. Only a single prefix is permitted. Use ns rather than mμs. The first syllable of the prefix retains its stress in compounds; thus, preferred pronunciations are kil' oh-mee-ter and mic' roh-mee-ter, not kil-lom' e-ter or mi-crom' e-ter. Factor 101 102 103 106 109 1012 1015 1018 1021 1024 Prefix deka hecto kilo mega giga tera peta exa zetta yotta Symbol da h k M G T P E Z Y Factor 10-1 10-2 10-3 10-6 10-9 10-12 10-15 10-18 10-21 10-24 Prefix deci centi milli micro nano pico femto atto zepto yocto Symbol d c m μ n p f a z y Derived Units By combining SI base units, it is possible to derive all other units. A derived unit may correspond to more than one physical quantity, but a give physical quantity, although it may be expressed in terms of different units or different equivalent names for the same unit, has a unique dimension and a unique coherent unit within SI. Several of the most commonly used derived units of SI have been given special names. becquerel, Bq: activity of a radionuclide decaying at the rate of 1 transition per second; coulomb, C: quantity of electricity carried in one second by a current of 1 ampere farad, F: capacitance of a capacitor between the plates of which there appears a potential difference of 1 volt when it is charged by a quantity of electricity of 1 coulomb; gray, G: absorbed dose when 1 joule is imparted per kilogram of matter by ionizing radiation; henry, H: inductance of a closed circuit in which electromotive force of 1 volt is produced when the electric current in the circuit varies uniformly at the rate of 1 ampere per second; hertz, Hz: frequency of a periodic phenomenon, the period of which is 1 second; joule, J: work done when the point of application of a force of 1 newton moves a distance of 1 meter in the direction of the force; newton, N: force that gives to a mass of 1 kilogram an acceleration of 1 meter per second squared. ohm, Ω: electric resistance between two points of a conductor (not being the seat of any electromotive force) when a constant potential difference of 1 volt, applied to these points, produces in the conductor a current of 1 ampere; pascal, Pa: pressure or stress of 1 newton per square meter; tesla, T: magnetic flux density given by a magnetic flux of 1 weber per square meter; volt, V: difference of electrical potential between two points of a conducting wire carrying a current of 1 ampere when the power dissipated is 1 watt; watt, W: power that, in 1 second, gives rise to energy of 1 joule. weber, Wb: magnetic flux that, linking a circuit of 1 turn, would produce in it an electromotive force of 1 volt if it were reduced to zero at a uniform rate in 1 second. Table 2: Derived SI units with special names. Quantity Name Frequency Force Pressure Energy, work, quantity of heat Power, radiant flux Quantity of electricity, electric charge Potential difference, electric potential, electromotive force Capacitance Electric resistance Magnetic flux Magnetic flux density, magnetic field Inductance hertz newton pascal joule Symbol Expression in terms of basic units Hz sec-1 N kg•m/s2 Pa kg/m•s2 J m2•kg/s2 Expression in terms of derived units J/m N/m2, J/m3 N•m watt W coulomb C m2•kg/s3 A•s J/s volt V m2•kg/s3•A W/A, J/C farad ohm weber tesla F Wb T s4•A2/m2•kg m2•kg/s3•A2 m2•kg/s2•A kg/s2•A C/V V/A V•s Wb/m2 henry H m2•kg/s2•A2 Wb/A Ω