Spring 2014 Physics 196L Course Syllabus Instructor
advertisement

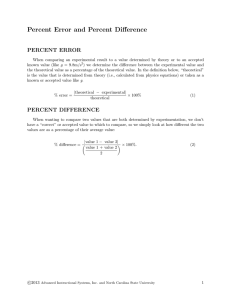
Spring 2014 Physics 196L Course Syllabus Instructor: Omair Zubairi Office: P-343A Office Hrs: Wed. 1:00-2:00 pm Section #: 01 Schedule #: 22431 Day/Time: Mon/1400-1640 Email: zubairi@rohan.sdsu.edu Class Webpage: www.rohan.sdsu.edu/~zubairi Requried Text: PHYSICS 182B/196L: PRINCIPLES OF PHYSICS LABORATORY MANUAL /AUTHOR: MURRAY FINEGOLD; VERSION 10.2-2012 Prerequisites: Credit or concurrent registration in Physics 196. Prerequisites (for PHYS 196): Physics 195 and Mathematics 151 Lab Fees: This lab requires a fee (to be paid at the cashier's office). Failure to pay this fee will result in your being dropped from this class. Introduction: Physics 196L is a one unit laboratory course designed to be taken concurrently with a Physics 196 lecture course. In this course, you will use the provided equipment to perform experiments in circuits and electromagnetism. These experiments are designed to reinforce the theoretical concepts presented in the lecture course. Attendance: Attendance is mandatory. Lab reports WILL NOT be accepted for experiments that you do not personally perform. This means that I will not accept reports based on your partner's data if you are not actually there. Grades: You will conduct 12 experiments during the semester. A lab report is required for all experiments. There will be no make up labs! The lab reports will be worth 75 % of your grade. We will also have 3 quizzes. I will let you know when the quizzes will be. The quizzes will be based on the previous few labs you have done. The quizzes will be worth 25 % of your grade. NOTE: I DO NOT GRADE BASED ON A CURVE IN THIS COURSE!! There is no final exam in this course. Grading Scale: A > 90; A- > 87; B+ > 83; B > 78; B- > 75; C+ > 70; C > 60; D > 50; F < 50 Lab Reports: Each report must have your name and your partner’s name. You will turn in the reports that are at the end of the lab manual. All of your calculations will be on a separate sheet(s) of paper. You will attach these extra sheets of paper with your report. All graphs will be done in excel or some other computer program(s) and will also be attached at the very end of your report. You will also be graded on your graphs. All questions I assign must be answered in complete sentences. You may answer the questions in the section of your reports listed “questions.” Lab reports will be due the following week. Late lab reports are NOT accepted. Cheating: Cheating will not be tolerated. The minimum penalty is an automatic FAIL in this class and you will be reported to the university administration. This means that you may not turn in a lab report for an experiment that you did not actually perform. Lab Schedule: The lab schedule is given here and is also provided in the 196L lab manual. Spring 2013: 196L Lab Schedule (CONTENT ARE TENTATIVE) Date: 27-Jan Exp. # Experiment No Experiment – Lab Orientation 3-Feb 1 Electric Field in a Capacitor 10-Feb 2 Parallel Plate Capacitors 17-Feb 3 Series & Parallel Capacitors 24-Feb 4 Electric Circuits 3-Mar 5 Current & Voltage 10-Mar 6 Series & Parallel Circuits 17-Mar 7 Intro. to Oscilloscope 24-Mar 8 Magnetic Fields of Coil Configurations 31-Mar --------- SPRING BREAK –NO LAB 7-Apr 9 Inductance & Faraday’s Law 14-Apr 10 RC Time Constants 21-Apr 11 LR Circuits 28-Apr 12 Resonance in LRC Circuits Things you need to keep in mind: • We frequently need to calculate percent error and percent difference. o Percent Error – Used to calculate the relative error between an experimental value and a theoretical value: measured − theoretical ! %error = ×100% It’s ok to get a theoretical NEGATIVE % error. ! € +/- % tells you whether your experimental values are high or low, relative to the theoretical value. o Percent Difference/Unbiased % Error – To be used when you only have measured or experimental values (i.e. there are no theoretical values to compare your data to). ⎛ ⎞ ⎜ value1 − value2 ⎟ ⎟ × 100% ALWAYS POSITIVE! ! % Difference = ⎜ ⎜ value1 + value2 ⎟ ⎜ ⎟ 2 ⎝ ⎠ ! Equivalently, if I use the rules for dividing fractions, ⎛ value1 − value2 ⎞ ⎟ × 200% %Difference = ⎜⎜ ⎟ value 1 + value 2 ⎝ ⎠ Notice that we are taking the absolute value. Therefore, it is NOT possible to get a negative percent difference. • • We will be working in SI units. You will always need to work in terms of kg, m, Newtons (N), etc. Below are a few of the common units and constants you will be using in this lab. Not all are listed here. Electron Mass: 9.11x10-31 kg Electric Charge: 1.602x10-19 C Coulomb Constant: k=8.99x109 N m2/C2 Force: 1N=kg m/s2 Energy: 1J=Nm Potential: 1V =J/C=Nm/C NOTE: I reserve the right to make any changes necessary to this syllabus.