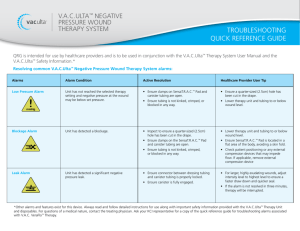
DISCLAIMER: This is intended for use as a supplement. Please refer to the Instructions for Use and User Manual for full safety information.
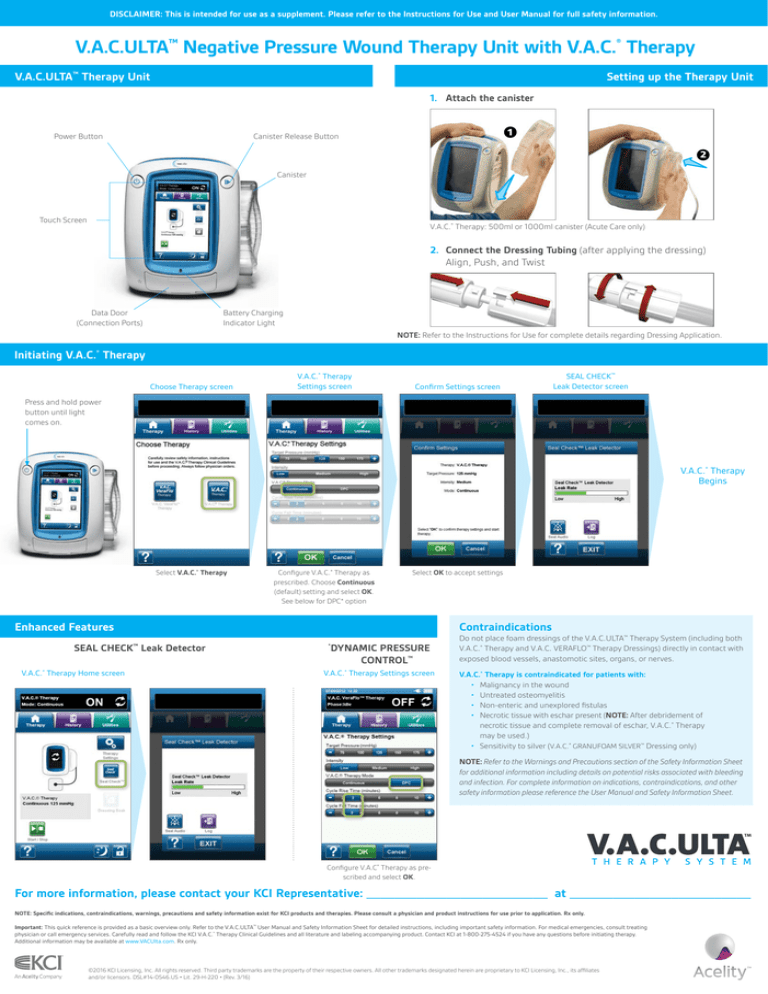
V.A.C.ULTA™ Negative Pressure Wound Therapy Unit with V.A.C.® Therapy
V.A.C.ULTA™ Therapy Unit
Setting up the Therapy Unit
1. Attach the canister
Power Button
Canister Release Button
22
Canister
Touch Screen
V.A.C.® Therapy: 500ml or 1000ml canister (Acute Care only)
2. Connect the Dressing Tubing (after applying the dressing)
Align, Push, and Twist
Data Door
(Connection Ports)
Battery Charging
Indicator Light
NOTE: Refer to the Instructions for Use for complete details regarding Dressing Application.
Initiating V.A.C.® Therapy
Choose Therapy screen
V.A.C.® Therapy
Settings screen
Confirm Settings screen
SEAL CHECK™
Leak Detector screen
Press and hold power
button until light
comes on.
V.A.C.® Therapy
Begins
Select V.A.C.® Therapy
Configure V.A.C.® Therapy as
prescribed. Choose Continuous
(default) setting and select OK.
See below for DPC* option
Select OK to accept settings
Enhanced Features
Contraindications
SEAL CHECK Leak Detector
™
V.A.C.® Therapy Home screen
DYNAMIC PRESSURE
CONTROL™
*
V.A.C.® Therapy Settings screen
Do not place foam dressings of the V.A.C.ULTA™ Therapy System (including both
V.A.C.® Therapy and V.A.C. VERAFLO™ Therapy Dressings) directly in contact with
exposed blood vessels, anastomotic sites, organs, or nerves.
V.A.C.® Therapy is contraindicated for patients with:
• Malignancy in the wound
• Untreated osteomyelitis
• Non-enteric and unexplored fistulas
• Necrotic tissue with eschar present (NOTE: After debridement of
necrotic tissue and complete removal of eschar, V.A.C.® Therapy
may be used.)
• Sensitivity to silver (V.A.C.® GRANUFOAM SILVER™ Dressing only)
NOTE: Refer to the Warnings and Precautions section of the Safety Information Sheet
for additional information including details on potential risks associated with bleeding
and infection. For complete information on indications, contraindications, and other
safety information please reference the User Manual and Safety Information Sheet.
Configure V.A.C® Therapy as prescribed and select OK.
For more information, please contact your KCI Representative: _________________________ at _________________________
NOTE: Specific indications, contraindications, warnings, precautions and safety information exist for KCI products and therapies. Please consult a physician and product instructions for use prior to application. Rx only.
Important: This quick reference is provided as a basic overview only. Refer to the V.A.C.ULTA™ User Manual and Safety Information Sheet for detailed instructions, including important safety information. For medical emergencies, consult treating
physician or call emergency services. Carefully read and follow the KCI V.A.C.® Therapy Clinical Guidelines and all literature and labeling accompanying product. Contact KCI at 1-800-275-4524 if you have any questions before initiating therapy.
Additional information may be available at www.VACUlta.com. Rx only.
©2016 KCI Licensing, Inc. All rights reserved. Third party trademarks are the property of their respective owners. All other trademarks designated herein are proprietary to KCI Licensing, Inc., its affiliates
and/or licensors. DSL#14-0546.US • Lit. 29-H-220 • (Rev. 3/16)
DISCLAIMER: This is intended for use as a supplement. Please refer to the Instructions for Use and User Manual for full safety information.
V.A.C.ULTA™ Negative Pressure Wound Therapy Unit with V.A.C. VERAFLO™ Therapy
Setting up the Therapy Unit
V.A.C.ULTA™ Therapy Unit
11
Power Button
1. Attach the V.A.C. VERALINK™ Cassette
V.A.C.
V.A.C.
VeraLink™
VeraLink™
Cassette
Cassette
PivotPivot
Connection
Connection
22
Solution
Container Arm
22
1
Solution Bag
33
V.A.C. VeraLink™ Cassette
Pivot Connection
2
2. Extend the Solution Arm
2
3 3
3
4 4
3. Attach the 500ml or
1000ml Canister
3
2
4. Spike and Hang the Solution
Container Bag/Bottle
2
2
2
3
5. Connect the Dressing Tubing (after applying the dressing)
Align, Push, and Twist
3
Battery Charging
Indicator Light
Data Door
(Connection Ports)
Do not place foam dressings of the V.A.C.ULTA™ Therapy System (including both V.A.C.® Therapy and
V.A.C. VERAFLO™ Therapy Dressings) directly in contact with exposed blood vessels, anastomotic sites,
organs, or nerves.
4
2
Canister
Contraindications
3
4
Touch Screen
Instillation Cassette
(V.A.C. VERALINK™
Cassette)
2
2
Canister Release Button
2 2
3
V.A.C.® Therapy and V.A.C. VERAFLO™ Therapy are contraindicated for patients with:
• Malignancy in the wound
• Untreated osteomyelitis
• Non-enteric and unexplored fistulas
• Necrotic tissue with eschar present (NOTE: After debridement of necrotic tissue and
complete removal of eschar, V.A.C.® Therapy may be used.)
• Sensitivity to silver (V.A.C.® GRANUFOAM SILVER™ Dressing only)
NOTE: Refer to the Warnings and Precautions section of the Safety Information Sheet for additional information including details on potential risks associated with bleeding and
infection. For complete information on indications, contraindications, and other safety information please reference the User Manual and Safety Information Sheet.
Additional Contraindications Specific to V.A.C. VERAFLO™ Therapy:
• Do not use V.A.C.® Dressings with Octenisept®*, hydrogen peroxide or solutions that are alcohol-based or contain alcohol.
• Do not deliver fluids to the thoracic or abdominal cavity due to the potential risk to alter core body temperature and the potential for fluid retention within the cavity.
• Do not use V.A.C. VERAFLO™ Therapy unless the wound has been thoroughly explored due to
the potential for inadvertent instillation of topical wound solutions to adjacent body cavities.
Connect the Instillation Line and then the V.A.C.® Therapy Line
NOTE: Refer to the Instructions for Use for complete details regarding Dressing Application.
Initiating V.A.C. VERAFLO™ Therapy
Not available in the United States. Brand name referenced is not a trademark of KCI, its affiliates, or licensors.
*
V.A.C. VERAFLO
Settings screen
™
Choose Therapy screen
Confirm Settings screen
Press and hold power
button until light
comes on.
V.A.C. VERAFLO™
Therapy Begins
Select V.A.C. VERAFLO™ Therapy
Confirm V.A.C. VERAFLO™ Therapy
as prescribed and select OK.
Select OK to accept settings
Select V.A.C. VERAFLO™ Therapy
Configure V.A.C. VERAFLO™ Therapy
as prescribed and select OK.
Select OK to accept settings
Enhanced Features
Fill Assist (with test cycle)
SEAL CHECK™ Leak Detector
Dressing Soak
For more information, please contact your KCI Representative: _________________________ at _________________________
NOTE: Specific indications, contraindications, warnings, precautions and safety information exist for KCI products and therapies. Please consult a physician and product instructions for use prior to application. Rx only.
Important: This quick reference is provided as a basic overview only. Refer to the V.A.C.ULTA™ User Manual and Safety Information Sheet for detailed instructions, including important safety information. For medical emergencies, consult treating
physician or call emergency services. Carefully read and follow the KCI V.A.C.® Therapy Clinical Guidelines and all literature and labeling accompanying product. Contact KCI at 1-800-275-4524 if you have any questions before initiating therapy.
Additional information may be available at www.VACUlta.com. Rx only.
©2016 KCI Licensing, Inc. All rights reserved. Third party trademarks are the property of their respective owners. All other trademarks designated herein are proprietary to KCI Licensing, Inc., its affiliates
and/or licensors. DSL#14-0546.US • Lit. 29-H-220 • (Rev. 3/16)