electronics
Review
Electrolytic Gated Organic Field-Effect Transistors for
Application in Biosensors—A Review
Denjung Wang, Vincent Noël and Benoît Piro *
Univ. Paris Diderot, Sorbonne Paris Cité, ITODYS, UMR 7086 CNRS, 15 rue J-A de Baïf, 75205 Paris Cedex 13,
France; denjung.wang@univ-paris-diderot.fr (D.W.); vincent.noel@univ-paris-diderot.fr (V.N.)
* Correspondence: piro@univ-paris-diderot.fr; Tel.: +33-1-57-27-72-24
Academic Editors: Emil J. W. List-Kratochvil and Ruth Shinar
Received: 10 December 2015; Accepted: 16 February 2016; Published: 25 February 2016
Abstract: Electrolyte-gated organic field-effect transistors have emerged in the field of biosensors over
the last five years, due to their attractive simplicity and high sensitivity to interfacial changes, both
on the gate/electrolyte and semiconductor/electrolyte interfaces, where a target-specific bioreceptor
can be immobilized. This article reviews the recent literature concerning biosensing with such
transistors, gives clues to understanding the basic principles under which electrolyte-gated organic
field-effect transistors work, and details the transduction mechanisms that were investigated to
convert a receptor/target association into a change in drain current.
Keywords: electrolyte-gated organic field-effect transistors (EGOFET); biosensors
1. Introduction
A biosensor, defined by the International Union of Pure and Applied Chemistry (IUPAC), is “a
device which uses specific biochemical reactions mediated by isolated enzymes, immunosystems,
tissues, organelles or whole cells to detect chemical or biological compounds, usually by use of
electrical, thermal or optical signals” [1,2]. Biosensors are promising analytical tools for healthcare,
monitoring food toxins and pathogens, and environmental screening due to their unique characteristics
of affordability, portability, disposability, and simple construction [3]. From the first biosensor reported
(enzyme-coated oxygen electrode [4]), more and more biosensors were reported, mostly for medical
applications: blood gases, hemoglobin, glucose, calcium, urea and many other critical analyses.
Biosensors are also able to sense basic parameters such as humidity or pH, but also numerous types
of non-biological compounds such as heavy metals or small organic pollutant molecules such as
bisphenol A. However, until now, biosensors have not invaded the market. This may be due to a still
too high production cost, at least for most everyday life applications. With the coming of the Web of
Things, i.e., connected objects implementing sensors, along with a growing need for healthcare devices,
biosensors may at last fulfill their promises. For this, they must be better interfaced with electronics;
transistors as biosensing components may be the way to achieve this objective.
A transistor (a “transfer resistor”, because “it is a resistor or semiconductor device which can
amplify electrical signals as they are transferred through it from input to output terminals”, as defined
by the three winners of Nobel Prizes in Physics in 1956) is composed of a semiconductor material with
three or four terminals for connection to an external circuit. A voltage or current applied to one pair of
the transistor’s terminals (“gate” and “body”) changes the current through another pair of terminals
(“source” and “drain”).
Nowadays, the most popular transistor used is the field-effect transistor (FET) [5], even
if electrochemical transistors based of PEDOT:PSS, poly(ethylenedioxythiophene):poly(styrene
Electronics 2016, 5, 9; doi:10.3390/electronics5010009
www.mdpi.com/journal/electronics
Electronics 2016, 5, 9
2 of 24
sulfonate), are widely reported as well [6]. In the past few years, field-effect transistors have been
intensively investigated for biosensing applications, because of their natural integration into portable
electronic devices, but also because the field effect is capacitance-related, and this capacitance is known
to be very sensitive to surface changes. For example, the presence of guest molecules over one of the
gate/dielectric or dielectric/semiconductor interface would result in a shift of the conductance of the
semiconductor. However, the main drawback with conventional silicon-based transistors is the high
cost of silicon microlithography operated in clean room, which is prohibitive for disposable sensors.
Nevertheless, their sensitivities are high, characterized by subthreshold swings of ca. 70 mV/dec for
conventional inorganic MOSFETs and significantly lower values for non-conventional FETs such as
tunneling FETs (10–20 mV/dec), which are based on band-to band tunneling due to band bending
induced by charges immobilized at the interface between a semiconductor nanowire and a solution [7]).
Organic thin-film transistors (OTFTs) will probably never compete with inorganic ones in terms of
carrier mobility and operating frequency, and their subthreshold swing is often much higher, which
makes them intrinsically less sensitive up to now. However, organic semiconductors can be processed
at low cost, for example by use of printing techniques, and can be easily chemically modified to
adjust their properties, which is decisive for biosensors where the recognition elements have to be
attached [8].
2. General Concepts of Transistors
A field effect transistor (FET) is consists of three metallic conducting electrodes: source (S),
drain (D) and Gate (G), a very thin insulating layer (dielectric) and a semiconductor, the latter being
the active part of the device where charge carriers flow. Numerous geometries were described,
among which the gate could be on top of the semiconductor (top-gate) or at the bottom of the
semiconductor (bottom-gate). Similarly, the source and drain can contact the top (top-contact) or
the bottom (bottom-contact) of the semiconductor. The working principle of a FET is the following:
a voltage applied to the gate modifies the charge carrier density in the semiconductor in between
source and drain, which therefore modulates the source-drain current. There are two kinds of charge
carriers: electrons (e) and hole (h) for n or p type semiconductors, respectively. Dealing with organic
FETs (OFETs), electron donating organic semiconductors involving a high highest occupied molecular
orbital (HOMO) level are good candidates for p-type semiconductors, while electron-accepting ones
with low HOMO levels are used as n-type semiconductors.
In OFETs, the gate is separated from the semi-conductor by an insulator. Different dielectrics
can be used, e.g., oxides, polymers, self-assembled monolayers. When the gate is, for instance,
negatively polarized in a p-channel device, free holes in the semi-conductor are drawn toward the
semi-conductor-insulator interface to compensate an equivalent negative charge at the gate-insulator
interface (Figure 1a). When a negative voltage is applied between source and drain, holes are injected
from the source and current flows inside the channel. The OFET characteristics are greatly affected by
the properties of the dielectric-insulator interface or at grain boundaries. The first OFET was made
by Tsumura et al., in 1986, who reported a Ion /Ioff ratio (i.e., the ratio between the maximum current
flowing at saturation and the minimum current when the transistor is in its blocked state) of 100, and
charge carrier mobility of 10´5 cm2 ¨ V´1 [9], using a polythiophene semiconductor.
gate-insulator interface (Figure 1a). When a negative voltage is applied between source and drain,
holes are injected from the source and current flows inside the channel. The OFET characteristics are
greatly affected by the properties of the dielectric-insulator interface or at grain boundaries. The first
OFET was made by Tsumura et al., in 1986, who reported a Ion/Ioff ratio (i.e., the ratio between the
maximum current flowing at saturation and the minimum current when the transistor is in its
blocked
of 100, and charge carrier mobility of 10−5 cm2·V−1 [9], using a polythiophene3 of 24
Electronics
2016, state)
5, 9
semiconductor.
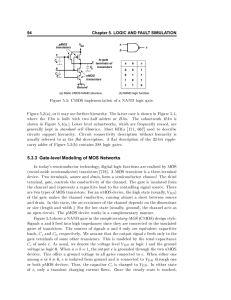
Figure 1. General scheme of an organic field-effect transistor (OFET) (a) and an electrolyte-gated
organic field-effect transistor (EGOFET) (b). Adapted from [8] with kind permission from Springer
Science+Business Media. © Springer-Verlag, 2011.
3. EGOFETs
Electrolyte-gated OFETs are significantly different from classical OFETs; indeed, the organic
semiconducting layer is in contact with an electrolyte instead of a classical dielectric. In EGOFETs,
the conductivity of the semiconducting channel is modulated by a solid or a liquid electrolyte put in
between the semiconductor and the gate. EGOFETs display much a higher gate capacitance (up to
~1000 higher) than other types of OFETs, which use traditional inorganic or organic non-electrolytic
dielectrics; as a consequence, biasing voltages used for EGOFETs are typically much smaller than those
necessary for OFETs (<1 V, versus >10 V or even higher). These two characteristics (electrolyte—possibly
water—and low potential) make EGOFETs ideal candidates for the next generation of biosensors,
particularly suitable for the detection and quantification of biological molecules inside aqueous
media. The extremely good sensing capabilities of EGOFETs rely on the possibility of properly
functionalizing the gate electrode by means of specific molecules or functional groups able to interact
with the target molecules present inside the electrolyte. The most common architecture is the top-gate,
bottom-contact configuration. The gate electrode is immersed in the electrolyte and source and
drain electrodes, isolated from the electrolyte, provide electrical contact to the channel (Figure 1b).
Actually, an EGOFET looks like an OECT (organic electrochemical transistor) [10–16]. However, in an
OECT, the on/off switch is produced by electron transfer from the electrolyte and the semiconductor
(doping/de-doping) [8], whereas only capacitive processes occur for EGOFETs but no charge transfer.
In an EGOFET made with a p-type semiconductor, upon positive polarization of the gate, the
anions of the electrolyte accumulate at the electrolyte/semiconductor interface while the cations
accumulate at the electrolyte/OSC interface, resulting in the formation of an electrical double layer
(EDL) at both interfaces. The EDL is composed of the Helmholtz layer (HL) and the diffuse layer (DL).
The HL is a monolayer of ions, whereas the DL is simply more concentrated in ions (either cations or
anions) than in the bulk electrolyte. In other words, the excess of ions decreases with the distance from
the interface. As shown on Figure 2, when the gate is (for example) negatively polarized, the excess
of electrons at the gate surface produces the accumulation of cations at the gate/electrolyte interface;
conversely, accumulation of anions at the electrolyte/semiconductor interface produces accumulations
of holes in the topmost layer of the semiconductor [17,18], which causes the OSC to become conducting.
It has been shown that a significant double layer can form even for very low operating potentials,
nevertheless sufficient to generate a locally high electrical field at the electrolyte/semiconductor
interface, and therefore a high charge carrier density [8].
The electrolyte used can be polymers [19–22], ionic liquids [23–25] or ionic gels [16,26–29].
Aqueous liquid electrolytes were also reported. For example, Kergoat et al. [10] first reported a
device gated with water (Figure 3). By replacing the gate dielectric by a simple water droplet, they
produced a transistor that entirely operates in the field-effect mode at voltages lower than 1 V. They
have shown (unpublished results) that the electrical characteristics do not depend on the pH in a range
between 4 and 8, which is well adapted to biomolecules.
distance from the interface. As shown on Figure 2, when the gate is (for example) negatively
polarized, the excess of electrons at the gate surface produces the accumulation of cations at the
gate/electrolyte interface; conversely, accumulation of anions at the electrolyte/semiconductor
interface produces accumulations of holes in the topmost layer of the semiconductor [17,18], which
causes the OSC to become conducting. It has been shown that a significant double layer can form
even
Electronics 2016,
5, 9for very low operating potentials, nevertheless sufficient to generate a locally high electrical
field at the electrolyte/semiconductor interface, and therefore a high charge carrier density [8].
Electronics 2016, 5, 9
Electronics 2016, 5, 9
4 of 24
4 of 23
4 of 23
Figure 2. Illustration of the compact and diffuse layers corresponding to OFET and EGOFET,
respectively. Adapted from [8] with kind permission from Springer Science+Business Media. ©
Springer-Verlag, 2011.
The electrolyte used can be polymers [19–22], ionic liquids [23–25] or ionic gels [16,26–29].
Figure
2. Illustration
of the compact
and diffuseFor
layers
corresponding
to et
OFET
and first
EGOFET,
Aqueous
liquid
electrolytes
also and
reported.
example,
Kergoat
al.to[10]
reported
a
Figure 2. Illustration
of the were
compact
diffuse layers
corresponding
OFET and
EGOFET,
respectively.
Adapted
from
[8]
with
kind
permission
from
Springer
Science+Business
Media.
©
device gated with water (Figure 3). By replacing the gate dielectric by a simple water droplet,
they
respectively.
Adapted 2011.
from [8] with kind permission from Springer Science+Business Media. ©
Springer-Verlag,
produced a transistor that entirely operates in the field-effect mode at voltages lower than 1 V. They
Springer-Verlag, 2011.
have shown
(unpublished results) that the electrical characteristics do not depend on the pH in a
The electrolyte used can be polymers [19–22], ionic liquids [23–25] or ionic gels [16,26–29].
range
between
4 and
8, which is
wellalso
adapted
to biomolecules.
Aqueous liquid
electrolytes
were
reported.
For example, Kergoat et al. [10] first reported a
device gated with water (Figure 3). By replacing the gate dielectric by a simple water droplet, they
produced a transistor that entirely operates in the field-effect mode at voltages lower than 1 V. They
have shown (unpublished results) that the electrical characteristics do not depend on the pH in a
range between 4 and 8, which is well adapted to biomolecules.
Figure 3. Scheme of the first water-gated EGOFET. The semiconductor was rubrene and the gate a
Figure 3.
Scheme
ofReproduced
the first water-gated
EGOFET.
TheWILEY-VCH
semiconductor
rubrene
and the gate
platinum
wire.
from [10]. Copyright
© 2010,
Verlagwas
GmbH
& Co. KGaA,
a platinum
wire.
Reproduced
from
[10].
Copyright
©
2010,
WILEY-VCH
Verlag
GmbH
& Co.
Weinheim.
KGaA, Weinheim.
Figure 3. Scheme of the first water-gated EGOFET. The semiconductor was rubrene and the gate a
This
result creates opportunities for sensor applications. Indeed, water is the natural
platinum wire. Reproduced from [10]. Copyright © 2010, WILEY-VCH Verlag GmbH & Co. KGaA,
environment
for biological receptors [30]. In this case, water must act both as the electrolyte and the
Weinheim.
This
result
creates opportunities for sensor applications. Indeed, water is the natural environment
media responsible for carrying the analytic sample, which is the current challenge to develop
for biological
receptors
[30]. In
this case,
water
must
act
bothIndeed,
as thewater
electrolyte
and
the media
EGOFET-based
biosensors.
However,
in such
conditions,
an electrochemical
doping
(i.e.,natural
insertion
This
result
creates
opportunities
for
sensor
applications.
is the
responsible
forsemiconductor
carrying
the analytic
sample,
which
ismay
the
current
challenge
develop
EGOFET-based
environment
for biological
receptors
[30].
In
this case,
water
must
act
both
as thetoelectrolyte
and
theand
into
the
of ions
from
the
electrolyte)
occur,
which
decreases
the
capacitance
responsible
for such
carrying
the analytic
sample,
which electrical
is the current
challenge
themedia
field
effect and in
therefore
degrades
the semiconductor
properties.
Thisto
isdevelop
the main
biosensors.
However,
conditions,
an
electrochemical
doping
(i.e.,
insertion
into the
EGOFET-based
biosensors. However,
in such often
conditions,
an electrochemical
doping
(i.e., [31,32];
insertionthis
reason
why
the
performances
of
EGOFET
decrease
dramatically
upon
use
semiconductor of ions from the electrolyte) may occur, which decreases the capacitance and the
into the semiconductor
of ions
electrolyte)
may occur, for
which
decreasesbythedeveloping
capacitance and
constitutes
an important
issuefrom
thatthemust
be addressed;
example,
highly
field effectthe
and
therefore
degrades
the semiconductor
electrical
properties.
Thisisisthe
themain
main reason
field
effect
and
therefore
degrades
the
semiconductor
electrical
properties.
This
hydrophobic polymeric OSC. However, some small analytes or even ions have no problems to
pass a
why thethin
performances
ofperformances
EGOFET
often
decrease
upon use
[31,32];
this
constitutes
an
reason
why thebarrier.
of EGOFET
decrease
dramatically
upon
use [31,32];
this of
hydrophobic
High crystallinity
ofoften
the dramatically
organic
semiconductor
can
suppress
diffusion
constitutes
anmust
important
issue that for
must
be addressed;
for example,
by developing
highlypolymeric
important
issue
that
be
addressed;
example,
by
developing
highly
hydrophobic
such species. Since most polymer films are semi-crystalline at best, this might be an aspect where
hydrophobic polymeric OSC. However, some small analytes or even ions have no problems to pass a
OSC. However,
analytes
even ions
have
problems
to pass
a thin hydrophobic
crystallinesome
films small
made from
smallormolecules
might
beno
superior
in terms
of blocking
and should be barrier.
thin hydrophobic barrier. High crystallinity of the organic semiconductor can suppress diffusion of
investigated.
Another
issue
that
must
be
addressed
is
their
high
subthreshold
swing,
typically
of
High crystallinity
of Since
the organic
semiconductor
can suppress
diffusion
ofbesuch
species.
such species.
most polymer
films are semi-crystalline
at best,
this might
an aspect
whereSince most
several hundreds of millivolts, which impede their sensitivity. A strategy may be to switch from
crystalline films made from small molecules might be superior in terms of blocking and should be
investigated. Another issue that must be addressed is their high subthreshold swing, typically of
several hundreds of millivolts, which impede their sensitivity. A strategy may be to switch from
Electronics 2016, 5, 9
5 of 24
polymer films are semi-crystalline at best, this might be an aspect where crystalline films made from
small molecules might be superior in terms of blocking and should be investigated. Another issue that
must be addressed is their high subthreshold swing, typically of several hundreds of millivolts, which
impede their sensitivity. A strategy may be to switch from classical OSC to graphene-based materials,
e.g., graphene or reduced graphene oxide, which has already been reported [33–46], but this will not
be discussed here. Readers who are interested in grapheme-based EGOFETs may report on the review
from Yan et al., 2013, which dealt with graphene-based transistors for biological sensors [47]. However,
graphene has been shown to have low sensitivity due to lack of bandgap. For this reason, other
electrolyte-gated inorganic FETs are also promising, for example based on MoS2 semiconductor [48]. In
EGOFETs, the gate material also influences the figures of merit of the devices, particularly the threshold
potential VT [49,50]. However, most of the transistor characteristics come from the semiconducting
material itself and from the quality of the contact between the source and drain electrodes and the
OSC. This quality noticeably depends on the way the semiconductor is deposited.
4. Semiconducting Materials
4.1. Deposition Techniques
Several deposition techniques were described; among them: spin coating, vacuum thermal
deposition, printing and spray deposition. Spin-coating and drop-casting remain the most popular in
laboratory, whereas inkjet printing and spray deposition are preferentially used in industry; vacuum
thermal evaporation is used in both environments.
4.1.1. Vacuum Thermal Evaporation
For vacuum thermal evaporation, both the semiconducting material and the substrate are placed
in high vacuum. The OSC is thermally sublimated then is condensed on the cold substrate to form a
film, the thickness of which being controlled by the deposition time and the temperature [51,52].
4.1.2. Spin-Coating
Spin-coating consists of spinning the substrate onto which a solution containing the
semiconducting material is deposited. Spinning produces a centrifugal force that homogeneously
spreads the solution and forms a very thin film over the substrate; its thickness is a balance between
the centrifugal force and the solution viscosity. During this operation, the solvent evaporates, leaving
a thin film of semiconducting material. An additional annealing step is needed to crystalize the
semiconductor molecules, which improve the electrical performances [53,54]. Very roughly, the film
thickness depends on the concentration of the solution (the more concentrated, the thicker), the rotation
speed (the faster, the thinner) and the evaporation rate of the solvent (the faster, the thicker). Compared
to vacuum thermal evaporation, the advantage of spin-coating is that it does not need heating, which
avoids thermal degradation of semiconductors. The main limitation is that all semiconductors cannot
dissolve in volatile solvents.
4.1.3. Inkjet Printing and Spray Deposition
Inkjet printing is a non-contact solution-processed technique which allows non-lithographic
patterning, for a lateral resolution as small as 5 µm, however depending on substrate preparation [55,56].
Inkjet printing is an additive technology and is therefore much less wasteful than other solution-based
methods using subtractive patterning methods; it generally ensures a better electrical contact with the
underlying electrode/material than the vacuum thermal evaporation [57] but it has the disadvantages
of poor lateral resolution compared to lithography [31,58–60].
Spray deposition is another non-contact solution-based method. In this case, instead of thermal
of piezoelectric propulsion of the ink, the liquid is pumped and guided through a gas flow to the
Electronics 2016, 5, 9
6 of 24
substrate. Like for inkjet-printing, the ink arrives at the substrate as micrometric droplets. For this
reason, a challenge for both techniques is to achieve uniform films [52,61].
4.2. Semiconducting Materials
Electronics 2016, 5, 9
6 of 23
4.2.1. Generalities
4.2. Semiconducting Materials
It is remarkable to note that no rationale has been published concerning chemical stability
of OSCs applicable
in EGOFETs. One may keep in mind that the highest occupied molecular
4.2.1. Generalities
orbital (HOMO)
and lowest
unoccupied
molecular
orbital
(LUMO)
levels
of the
OSCofshould not
It is remarkable
to note
that no rationale
has been
published
concerning
chemical
stability
OSCs applicable
in EGOFETs.
One mayanother
keep in mind
the highestparameter
occupied molecular
allow electrolyte
oxidation
or reduction;
verythat
important
is thatorbital
the electrolyte
(HOMO)
and lowest
orbital (LUMO)
levels
of the OSC should
allow Buth et al.
should not
penetrate
into unoccupied
the OSC, molecular
which would
become
electroactive.
Evennot
though
electrolyte oxidation or reduction; another very important parameter is that the electrolyte should
used α-sexithiophene
(α6T) in 2011 and 2012 to make an EGOFET-based biosensor [11,62],
not penetrate into the OSC, which would become electroactive. Even though Buth et al. used
most EGOFET
biosensors
were
reported
withan poly(3-hexylthiophene)
(P3HT)
as the organic
α-sexithiophene (α6T) in
2011 and
2012 to make
EGOFET-based biosensor [11,62],
most EGOFET
biosensors
were reported with
poly(3-hexylthiophene)
(P3HT) as the
organic semiconductors
semiconductors
[8,32,60,63–72].
In recent
years, thienothiophene
copolymers
[73], especially the
[8,32,60,63–72]. In recent years, thienothiophene copolymers
[73],also
especially
the due to a
poly(2,5-bis(3-alkylthiophen-2-yl)thieno[3,2-b]-thiophene)
(pBTTT) was
considered,
poly(2,5-bis(3-alkylthiophen-2-yl)thieno[3,2-b]-thiophene) (pBTTT) was also considered, due to a
better stability
in humid environment or even in water (Figure 4) [74–77].
better stability in humid environment or even in water (Figure 4) [74–77].
C6H13
S
S
S
S
S
S
H3C
α6T
S
CH3
n
P3HT
H29C16
S
H3C
S
S
CH3
n
S
C16H29
pBTTT
Figure 4. Most reported semiconducting oligomers and polymers in EGOFET biosensors.
Figure 4. Most reported semiconducting oligomers and polymers in EGOFET biosensors.
4.2.2. Poly(3-hexylthiophene) (P3HT)
4.2.2. Poly(3-hexylthiophene)
(P3HT)
P3HT is the most common
OSC used during the last decade [19]. Kline et al. analyzed a series of
regioregular polythiophenes on an OTFT device. They found that the crystallinity and molecular
P3HTweight
is theare
most
used during
the last
decade
[19]. from
Klinetheet well-defined
al. analyzed a series
twocommon
important OSC
parameters
on the hole
mobility,
resulting
of regioregular
polythiophenes
on an
OTFT
device.3-hexylthiophene
They found that
and molecular
molecular
architecture of P3HT
[78].
For example,
units the
maycrystallinity
polymerize through
head-to-tail,
head-to-head
or
tail-to-tail
couplings;
the
percentage
of
head-to-tail
coupling
represents
weight are two important parameters on the hole mobility, resulting from the well-defined molecular
the degree of regioregularity of P3HT, which is critical to the electronic properties of the material.
architecture of P3HT [78]. For example, 3-hexylthiophene units may polymerize through head-to-tail,
High-performance OFETs based on highly regioregular P3HT were successfully fabricated by
head-to-head
or tail-to-tail
percentage
head-to-tail
represents
the degree of
solution
process andcouplings;
showed holethe
mobilities
higherof
than
0.1 cm2/Vs coupling
and very high
on/off ratios
5
regioregularity
of P3HT, which is critical to the electronic properties of the material. High-performance
(>10 ) [79].
If P3HT
deposited by P3HT
spin-coating,
the best solvent
is chloroform,
due to
its rapid
OFETs based on
highlyisregioregular
were successfully
fabricated
by solution
process
and showed
evaporation which limits crystallization
during the spin-coating process 5[79,80]; this impose a
2
hole mobilities higher than 0.1 cm /Vs and very high on/off ratios (>10 ) [79].
thermal post-treatment process able to favor crystallization and π−π stacking of the P3HT backbone.
If P3HT
is deposited
byresults
spin-coating,
bestformed
solvent
is chloroform,
due5).toIt its rapid
It has been
shown that this
in a lamellar the
structure
by interchain
stacking (Figure
haswhich
also been
shown
that the hole during
mobility the
varies
by two orders
of magnitude
on a thermal
evaporation
limits
crystallization
spin-coating
process
[79,80];depending
this impose
orientation
(parallel
or
normal
to
the
substrate)
of
the
lamellae
[53,54,81–86],
the
normal
one
giving
post-treatment process able to favor crystallization and π´π stacking of the P3HT backbone. It has been
the highest mobility.
shown that this results in a lamellar structure formed by interchain stacking (Figure 5). It has also been
shown that the hole mobility varies by two orders of magnitude depending on orientation (parallel or
normal to the substrate) of the lamellae [53,54,81–86], the normal one giving the highest mobility.
Electronics 2016, 5, 9
Electronics 2016, 5, 9
7 of 24
7 of 23
Electronics 2016, 5, 9
7 of 23
Figure 5. Lamelar structure of poly(3-hexylthiophène) (P3HT), normal or parallel to the substrate,
Figure 5. respectively.
Lamelar structure
of poly(3-hexylthiophène)
(P3HT),
normal
orcopyright,
parallel1999.
to the substrate,
Reprinted from
[54] by permission from Macmillan
Publishers
Ltd.
respectively. Reprinted from [54] by permission from Macmillan Publishers Ltd. copyright, 1999.
According to the work of Alberga et al. [53], spin-coated then annealed P3HT displays three
different zones: an interfacial zone close to the substrate (thickness of 10 Å), an intermediate zone 30
According
the
of Alberga interfacial
et al. [53],
spin-coated
then the
annealed
P3HT
displays
three
Å thick to
atop
andwork
an OSC/electrolyte
zone
over this distance;
first layers
display
the
highest degree
of order [53,87–89].
different zones:
an interfacial
zone close to the substrate (thickness of 10 Å), an intermediate zone
Some
were proposed
to
improve the
performance
and/or
of P3HT.
Figure
5.strategies
Lamelar
structure
of poly(3-hexylthiophène)
(P3HT),
normal
orbiocompatibility
parallel
to first
the substrate,
30 Å thick atop
and
an OSC/electrolyte
interfacial
zone
over this
distance;
the
layers
display the
For
example, Reprinted
Liu et al.from
printed
P3HT
above
another
terrylene
semiconductor
to1999.
make a
respectively.
[54]
by
permission
from
Macmillan
Publishers
Ltd.
copyright,
highest degree of order [53,87–89].
heterojunction of semiconductor [57]. Thiemann et al. spray-coated a layer of ion gel based of
Somesilane-ionic
strategies
were
proposed
to improve
the performance
and/or
of P3HT.
P3HT
obtain
properties
such
as low biocompatibility
modulus,
solution
Accordingliquids
to theon
work
of to
Alberga
etadvantageous
al. [53], spin-coated
then
annealed
P3HT
displays
three
processability
and
high specific
Magliulo
et al.
modified
the
of P3HT
by 30
For example,
Liu
et al.an
printed
P3HT
above
terrylene
semiconductor
to make
a heterojunction
different
zones:
interfacial
zonecapacitance
close toanother
the[61].
substrate
(thickness
of 10 Å),
ansurface
intermediate
zone
plasma-enhanced
vapor
chemical
deposition
(PE-CVD)
of this
a mixture
ethylene,
acid
andthe liquids
of semiconductor
Thiemann
et al.
spray-coated
a layer
of
ionofgel
based
of silane-ionic
Å thick
atop[57].
and an
OSC/electrolyte
interfacial
zone over
distance;
the
first acrylic
layers
display
argondegree
in a 1:3:1
ratio,[53,87–89].
which increased the –COOH content at the surface, eventually used to
highest
of order
on P3HT
to obtain
advantageous
properties such as low modulus, solution processability and high
covalently graft phospholipids on the OSC, themselves used to bind biological molecules [64,71,90].
Some strategies
were
proposed
to improve thethe
performance
and/or
biocompatibility
of P3HT. vapor
specific capacitance
[61].
Magliulo
et al.
surface
of
P3HT
bytoplasma-enhanced
Kergoat et al. [91], Sinno et al. [59]
andmodified
Toss et al. [92] used
chemical
reactions
bind P3HT with
For example, Liu et al. printed P3HT above another terrylene semiconductor to make a
another polymer
to make of
new
semiconductor
polymer blends
improved
stability,
chemical deposition
(PE-CVD)
a mixture
of ethylene,
acrylicwith
acid
and argon
in aelectronic
1:3:1 ratio, which
heterojunction of semiconductor [57]. Thiemann et al. spray-coated a layer of ion gel based of
performances
and
biocompatibility.
Suspène
et
al.
used
peptidic
coupling
to
directly
graft
biotin
on
increased
the –COOH
content
at the
eventually used
to covalently
graft
phospholipids
silane-ionic
liquids
on P3HT
to surface,
obtain advantageous
properties
such as low
modulus,
solution on the
P3HT in order to make a proof-of-concept streptavidin or avidin sensor [93]. All these approaches
OSC, themselves
used
tohigh
bind
biological
molecules
[64,71,90].
etthe
al. surface
[91], Sinno
et al.by[59] and
processability
andcompromises
specific
capacitance
[61]. Magliulo
et al.Kergoat
modified
of P3HT
impose making
between
the electrical
and immobilization
properties.
plasma-enhanced
vaporreactions
chemical to
deposition
(PE-CVD)
of a mixture
of ethylene,
acrylic
and
Toss et al.
[92] used chemical
bind P3HT
with another
polymer
to make
newacid
semiconductor
argon
in
a
1:3:1
ratio,
which
increased
the
–COOH
content
at
the
surface,
eventually
used
to
4.2.3.
Poly(2,5-bis(3-alkylthiophen-2-yl)thieno[3,2-b]-thiophene)
(pBTTT)
polymer blends with improved stability, electronic performances and biocompatibility. Suspène
et al.
covalently graft phospholipids on the OSC, themselves used to bind biological molecules [64,71,90].
used peptidic coupling
to isdirectly
biotin
on semiconductor
P3HT in order
to make
proof-of-concept
streptavidin
Even if P3HT
certainlygraft
the mostly
used
in OFETs,
its a
instability
is well known,
Kergoat et al. [91], Sinno et al. [59] and Toss et al. [92] used chemical reactions to bind P3HT with
which obviously
limits
its approaches
utilization for biosensors
[94–96]. pBTTT
has recentlybetween
attracted attention
or avidin
sensor
[93]. All
these
impose
making
compromises
the electrical and
another
polymerpromising
to make new
semiconductor semiconducting
polymer blends polymer.
with improved
as another
solution-processed
pBTTTstability,
shows electronic
better
immobilization
properties.
performances and biocompatibility. Suspène et al. used2 peptidic coupling to directly
2 graft biotin on
performances than P3HT (hole mobility up to 1.0 cm /Vs for pBTTT versus 0.1 cm /Vs for P3HT
P3HT
in order
to make a proof-of-concept
streptavidin
or avidin
sensorenvironment
[93]. All these
approaches
[97,98]),
less electrochemical
doping and much
better stability
in aqueous
[53,99].
4.2.3. Poly(2,5-bis(3-alkylthiophen-2-yl)thieno[3,2-b]-thiophene)
(pBTTT)
imposeLiquid-crystalline
making compromises
between
the used
electrical
and immobilization
pBTTT
was firstly
as semiconductor
layer in properties.
liquid-gated structure by
Naim et al. [100]; Chao et al. show that pBTTT has a similar lamellar structure than P3HT [101];
Even
if P3HT
is certainly the mostly used semiconductor in
OFETs, its instability is well known,
4.2.3.
Poly(2,5-bis(3-alkylthiophen-2-yl)thieno[3,2-b]-thiophene)
(pBTTT)hundreds of nm [75,96,98]
however,
pBTTT has larger crystalline domains extended over several
which obviously
limits
its
utilization
for
biosensors
[94–96].
pBTTT
recently attracted attention as
(Figure 6). This probably explains why pBTTT has better performances has
[102,103].
Even if P3HT is certainly the mostly used semiconductor in OFETs,
its instability is well known,
another promising solution-processed semiconducting polymer. pBTTT shows better performances
which obviously limits its utilization for
biosensors [94–96]. pBTTT has recently
attracted attention
than P3HT
(hole mobility
upsolution-processed
to 1.0 cm2 /Vs for
pBTTT versuspolymer.
0.1 cm2 /Vs
for shows
P3HT [97,98]),
less
as another
promising
semiconducting
pBTTT
better
2
2
electrochemical
doping
and
much
better
stability
in
aqueous
environment
[53,99].
performances than P3HT (hole mobility up to 1.0 cm /Vs for pBTTT versus 0.1 cm /Vs for P3HT
[97,98]), less electrochemical
doping
and used
much better
stability in aqueous
environment
[53,99].structure by
Liquid-crystalline
pBTTT was
firstly
as semiconductor
layer
in liquid-gated
Liquid-crystalline
pBTTT
was
firstly
used
as
semiconductor
layer
in
liquid-gated
by [101];
Naim et al. [100]; Chao et al. show that pBTTT has a similar lamellar structure structure
than P3HT
Naim et al. [100]; Chao et al. show that pBTTT has a similar lamellar structure than P3HT [101];
however, pBTTT has larger crystalline domains extended over several hundreds of nm [75,96,98]
however, pBTTT has larger crystalline domains extended over several hundreds of nm [75,96,98]
(Figure (Figure
6). This6).probably
explains why pBTTT has better performances [102,103].
This probably explains why pBTTT has better performances [102,103].
Figure 6. SEM image of (a) P3HT and (b) pBTTT crystalline structure. Reproduced from [97].
Creative Commons Attribution License (CC BY).
Figure 6. SEM image of (a) P3HT and (b) pBTTT crystalline structure. Reproduced from [97].
Figure 6.
SEM image
of (a)
P3HT and
(b) pBTTT
Creative
Commons
Attribution
License
(CC BY).crystalline structure. Reproduced from [97]. Creative
Commons Attribution License (CC BY).
Electronics 2016, 5, 9
Electronics 2016, 5, 9
8 of 24
8 of 23
The best solvent for spin-coating pBTTT is chlorobenzene. It was shown that, because the boiling
The best solventisfor
spin-coating
pBTTTsolvent
is chlorobenzene.
wasremain
shown after
that, because
the and
point of chlorobenzene
high,
few residual
molecules Itmay
annealing,
boiling point of chlorobenzene is high, few residual solvent molecules may remain after annealing,
these solvent molecules may behave as plasticizer, increase free space between chains thus facilitate
and these solvent molecules may behave as plasticizer, increase free space between chains thus
orientation and crystallization [53,98,102].
facilitate orientation and crystallization [53,98,102].
5. Biosensors with EGOFETs
5. Biosensors with EGOFETs
Hereafter
are reviewed
the various
EGOFET-based
biosensors
Hereafter
are reviewed
the various
EGOFET-based
biosensorsreported
reportedininthe
theliterature
literature since
since 2012.
2012.
5.1. α-Sexithiophene (α6T)-Based EGOFET Sensor for Detection of Enzyme Activity
5.1. α-Sexithiophene
(α6T)-Based
EGOFET
Sensor for
Detection
Enzymesolution-gated
Activity
Buth
et al. described
in 2012 [11]
an EGOFET
which
theyofnamed
organic field-effect
Buth
et semiconductor
al. described in (α-sexithiophene)
2012 [11] an EGOFET
which
they
named by
solution-gated
organic
transistors.
The
surface
was
modified
–OH or –NH
2 groups,
field-effect
The semiconductor
(α-sexithiophene)
by –OH
or –NH2 The
leading
to a pH transistors.
sensor operating
in a pH range
relative to thesurface
pKa ofwas
the modified
added surface
functions.
groups,
leading
to added
a pH sensor
operatingunder
in a pH
to the
pKa of
the added
added surface
hydroxyl
groups
were
by oxidation
UV,range
and relative
the amine
groups
were
by grafting
functions.
The
hydroxyl
groups
were
added
by
oxidation
under
UV,
and
the
amine
2 and were
3-aminopropyltriethoxysilane. The on/off ratio at VDS = ´50 mV was between 10groups
103 , with
added by grafting 3-aminopropyltriethoxysilane.
The on/off ratio at VDS = −50 mV was between 102 ´1
´
2
2
´
1
´
1
a field-effect
mobility of 4 ˆ 10 cm ¨ V ¨ s . The authors found a sensitivity of 14 mV¨ pH .
and 103, with a field-effect mobility of 4 × 10−2 cm2·V−1·s−1. The authors found a sensitivity of 14
As a proof-of-concept,
they immobilized the penicillinase enzyme on the semiconductor and sensed
mV·pH−1. As a proof-of-concept, they immobilized the penicillinase enzyme on the semiconductor
penicillin
(Figure
7)
by
the
way of
an the
acid-base
titration.
They
foundThey
a detection
limit of ca.
5 µM.
and sensed penicillin
(Figure
7) by
way of an
acid-base
titration.
found a detection
limit
of At
´1 .
low penicillin
concentration,
the
sensitivity
of
the
device
was
ca.
80
µV¨
µM
ca. 5 μM. At low penicillin concentration, the sensitivity of the device was ca. 80 μV·μM−1.
Figure 7. (a) Schematic drawing of the transistor architecture and the different functionalizations
Figure 7. (a) Schematic drawing of the transistor architecture and the different functionalizations
investigated. (b) Transfer curves of untreated, oxidized and APTES-functionalized transistors,
investigated. (b) Transfer curves of untreated, oxidized and APTES-functionalized transistors,
measured at pH 5. (c) Threshold voltage shift of untreated, APTES-functionalized and UV-oxidized
measured
5. (c)ofThreshold
voltage
shift
of untreated,
APTES-functionalized
andgreen
UV-oxidized
time,
during
a penicillin
titration in PBS buffer. The
line
α6T asata pH
function
pH. (d) ID versus
α6T as
a
function
of
pH.
(d)
I
versus
time,
during
a
penicillin
titration
in
PBS
buffer.
The
D of a device functionalized with APTES and penicillinase, while thegreen
corresponds to the response
grey line
corresponds
to the
device
functionalized
with
APTES
and penicillinase,
while the grey
untreated
α6T. Adapted
from
[11]. Copyright
© 2012, WILEY-VCH
line depicts
the response
ID responseofofaan
line depicts
the ID &
response
of an
untreated α6T. Adapted from [11]. Copyright © 2012, WILEY-VCH
Verlag GmbH
Co. KGaA,
Weinheim.
Verlag GmbH & Co. KGaA, Weinheim.
5.2. P3HT-Based EGOFET Sensor for DNA Detection
5.2. P3HT-Based
DNA
A DNAEGOFET
EGOFETSensor
sensorfor
was
firstDetection
reported by Kergoat et al. [70] (Figure 8A), in which the
dielectric is constituted by a simple droplet of aqueous phosphate buffer saline (PBS, pH 7.2)
A DNA EGOFET sensor was first reported by Kergoat et al. [70] (Figure 8A), in which the dielectric
is constituted by a simple droplet of aqueous phosphate buffer saline (PBS, pH 7.2) solution. This
device allows operating at very low voltages (below 1 V). This renders possible the use of buffer
Electronics 2016, 5, 9
9 of 24
Electronics 2016, 5, 9
9 of 23
solution. This device allows operating at very low voltages (below 1 V). This renders possible the use
solutions
within
their electrochemical
stability window.
P3HT bearing
carboxylic
acid moieties
of buffer
solutions
within their electrochemical
stabilityAwindow.
A P3HT
bearing carboxylic
acid was
used moieties
to perform
covalent
ODN
(oligonucleotide)
grafting,
providing
that
they
are
modified
at
was used to perform covalent ODN (oligonucleotide) grafting, providing that they one
are end
with amodified
–COOHatgroup.
in the group.
outputChanges
characteristics
of thecharacteristics
device were of
observed
upon
one endChanges
with a –COOH
in the output
the device
wereDNA
observed upon
and after
DNA
(Table
1), ofvoltage
40 to 60shift
mV of
immobilization
andDNA
after immobilization
DNA hybridization
(Table
1),hybridization
of 40 to 60 mV
of gate
forgate
100 nM
100towards
nM target
DNA. This
shift
towards
negative
values
was attributed
theDNA
targetvoltage
DNA. shift
Thisfor
shift
negative
values
was
attributed
to the
negative
charge oftothe
negative
of the DNA
backbone.
The and
off current
was after
also modified
and decreased after
backbone.
Thecharge
off current
was also
modified
decreased
DNA immobilization.
ThisDNA
behavior
immobilization.
This
behavior
was
attributed
to
the
steric
hindrance
of
DNA
chains
that
eventually
was attributed to the steric hindrance of DNA chains that eventually prevents ion penetration into
prevents ion penetration into the bulk of the semiconductor (Figure 8B). Other experiments,
the bulk of the semiconductor (Figure 8B). Other experiments, obtained with various ionic strength,
obtained with various ionic strength, pointed out the importance of the Debye length that can screen
pointed out the importance of the Debye length that can screen negative DNA charges.
negative DNA charges.
(A)
(B)
Figure 8. (A) Schematic view of the EGOFET device. (B) Transfer characteristics after (a)
Figure
8. (A) Schematic view of the EGOFET device. (B) Transfer characteristics after (a) immobilization
immobilization of ODN probes and (b) a blank sample after soaking in a bath similar to (a) but
of ODN
probes
andTransfer
(b) a blank
sample
after soaking
in a(c)bath
similar to (a) target
but without
Transfer
without
ODN.
curves
for hybridization
with
a complementary
and (d)ODN.
a random
curves
for
hybridization
with
(c)
a
complementary
target
and
(d)
a
random
target.
Transfer
curves
target. Transfer curves for (e) a complementary target and (f) a random target in water instead of for
(e) a complementary
target
a random
target
in water
instead
PBS.
Adapted
[70] with
PBS. Adapted from
[70] and
with (f)
permission
from
Elsevier.
Copyright
© of
2011,
Elsevier
B.V.from
All rights
reserved.
permission from Elsevier. Copyright © 2011, Elsevier B.V. All rights reserved.
Electronics 2016, 5, 9
Electronics 2016, 5, 9
10 of 23
10 of 24
Table 1. Influence of probe grafting and target hybridization on device performance with phosphate
buffer saline (PBS) as electrolyte, and influence of probe hybridization with water as electrolyte.
Table 1. Influence of probe grafting and target hybridization on device performance with phosphate
Adapted from [70] with permission from Elsevier. Copyright © 2011, Elsevier B.V. All rights
buffer saline (PBS) as electrolyte, and influence of probe hybridization with water as electrolyte.
reserved.
Adapted from [70] with permission from Elsevier. Copyright © 2011, Elsevier B.V. All rights reserved.
ODN grafting (Figure 8a,b)
ODNODN
grafting
(Figure 8a,b)
probe
∆VG min / V
ODN
probe
∆VG min / V
Yes
−0.31 ± 0.05
Yes
´0.31
No
+0.16 ± 0.05 ˘ 0.05
No
+0.16 ˘ 0.05
DNA
hybrid. in PBS (Figure 8c,d)
DNA hybrid. in PBS (Figure 8c,d)
ODN
target
∆VG min ∆V
/ VG min / V
ODN
target
HIVHIV
−0.06 ± 0.02
´0.06 ˘ 0.02
RAND
´0.03 ˘ 0.03
RAND
−0.03 ± 0.03
DNA
hybrid.
in
H
O
(Figure
8e,f)
2
DNA hybrid. in H2O (Figure 8e,f)
ODN probe
∆V
/V
ODN probe
∆VGmin / V Gmin
HIV
´0.03 ˘ 0.02
HIV
−0.03 ± 0.02
RAND
´0.04 ˘ 0.07
RAND
−0.04 ± 0.07
Ioff (bare film) / Ioff (probe-modified film)
Ioff (bare11.1
off (probe-modified film)
film){±I3.7
0.5 ± 11.1
0.12˘ 3.7
0.5 ˘ 0.12
IoffI(probe-modified
film) / Ioff (hybridization)
off (probe-modified film){ Ioff (hybridization)
1.7 ± 1.7
0.45
˘ 0.45
1.3
˘
1.3 ± 0.28 0.28
I
I
off (probe-modified film){ off (hybridization)
Ioff (probe-modified
film) / Ioff (hybridization)
3.4 ˘ 1.5
3.4 ±1.04
1.5˘ 0.04
1.04 ± 0.04
Schmoltner et al. reported an EGOFET based on P3HT for which they investigated the effect of the
Schmoltner et al. reported an EGOFET based on P3HT for which they investigated the effect of
gate material (as in ref. [10]) and of the ionic strength of the electrolyte on the device performances [104].
the gate material (as in ref. [10]) and of the ionic strength of the electrolyte on the device
Magliulo et al. published in 2013 a work where they demonstrated PE-CVD of an hydrophilic
performances [104]. Magliulo et al. published in 2013 a work where they demonstrated PE-CVD of an
thin layer (plasma deposited ethylene/acrylic acid (pdEthAA)) carrying COOH groups on P3HT
hydrophilic thin layer (plasma deposited ethylene/acrylic acid (pdEthAA)) carrying COOH groups
(Figure 9) [71]. The thickness of the coating was particularly monitored because it should be minimized
on P3HT (Figure 9) [71]. The thickness of the coating was particularly monitored because it should
to avoid adverse effect on the EGOFET performance. A comparison between pristine P3HT and
be minimized to avoid adverse effect on the EGOFET performance. A comparison between pristine
pdEthAA/P3HT bilayer is given on Figure 10. It is also shown on this figure that the gate current
P3HT and pdEthAA/P3HT bilayer is given on Figure 10. It is also shown on this figure that the gate
(IG , red curves and right Y-scale) stay low, which demonstrates the field-effect mode of operation
current (IG, red curves and right Y-scale) stay low, which demonstrates the field-effect mode of
and excludes the electrochemical mode of operation; this shows that the hydrophobic layer fully
operation and excludes the electrochemical mode of operation; this shows that the hydrophobic
plays its role by impeding ion penetration in the OSC. In addition, the authors reported that chemical
layer fully plays its role by impeding ion penetration in the OSC. In addition, the authors reported
or biological species could be covalently immobilized on such functional hydrophobic/hydrophilic
that chemical or biological species could be covalently immobilized on such functional
bilayer, such as DNA probes (even if not described in their article).
hydrophobic/hydrophilic bilayer, such as DNA probes (even if not described in their article).
Figure
(B)(B)
Schematic
view
of an
Figure 9.
9. (A)
(A)Chemical
Chemicalstructure
structureofofP3HT,
P3HT,used
usedasasorganic
organicsemiconductor.
semiconductor.
Schematic
view
of
electrolyte
gated
organic
field-effect
transistor
with
the
pdEthAA
coating
deposited
by
PE-CVD
on
an electrolyte gated organic field-effect transistor with the pdEthAA coating deposited by PE-CVD
the
P3HT
layer.
Reproduced
from from
[71]. Copyright
© 2012,©WILEY-VCH
Verlag Verlag
GmbH GmbH
& Co. KGaA,
on the
P3HT
layer.
Reproduced
[71]. Copyright
2012, WILEY-VCH
& Co.
Weinheim.
KGaA, Weinheim.
Electronics 2016, 5, 9
11 of 23
Electronics 2016, 5, 9
11 of 24
Electronics 2016, 5, 9
11 of 23
Figure
10.
DS curves
of EGOFET
devicesfabricated
fabricated with
P3HT
and
pdEthAA/
P3HT
Figure
10.
–VIDS
DS–V
curves
of EGOFET
devices
with(a)
(a)pristine
pristine
P3HT
and
pdEthAA/
P3HT
Figure
10. IIDS
DS –VDS curves of EGOFET devices fabricated with (a) pristine P3HT and pdEthAA/ P3HT
bilayer
deposited
by PE-CVD
for:(b)(b)1515s;s;(c)
(c) 55 s;
s; and
and (d)
33s.s.LL= =2 2μm
and
WW
= 10,000
μm. μm.
bilayer
deposited
by
PE-CVD
for:
(d)
μm
and
=
10,000
bilayer deposited by PE-CVD for: (b) 15 s; (c) 5 s; and (d) 3 s. L = 2 µm and W = 10,000 µm. Reproduced
Reproduced from [71]. Copyright © 2012, WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Reproduced
from [71].©Copyright
© 2012, WILEY-VCH
Co. KGaA, Weinheim.
from [71]. Copyright
2012, WILEY-VCH
Verlag GmbHVerlag
& Co.GmbH
KGaA,&
Weinheim.
5.3. P3HT-Based EGOFET for Protein (Streptavidin) Detection
5.3.
Detection
5.3. P3HT-Based
P3HT-Based EGOFET
EGOFET for
for Protein
Protein (Streptavidin)
(Streptavidin) Detection
Suspene et al. reported in 2013 on the sensing of streptavidin with an EGOFET using a
Suspene
etof
reported
2013
on sensing
the sensing
of streptavidin
with
EGOFET
using a
copolymer
P3HT-COOH
biotinylated
P3HT
(Figure
11A)
the an
active
sensing
and
Suspene
et
al.al.P3HT,
reported
in in
2013
on and
the
of streptavidin
with
anas
EGOFET
using
a copolymer
copolymer
of
P3HT,
P3HT-COOH
and
biotinylated
P3HT
(Figure
11A)
as
the
active
sensing
semiconducting
material
[93]. The biotin-streptavidin
couple
as a proof-of-concept,
for and
of P3HT,
P3HT-COOH
and biotinylated
P3HT (Figure 11A)
aswas
the chosen
active sensing
and semiconducting
semiconducting
material
[93].
The
biotin-streptavidin
couple
was
chosen
as
a
proof-of-concept,
for
its extremely
low
dissociation constant.
Non-specific
interactions
reduced due tofor
theits
presence
material
[93]. The
biotin-streptavidin
couple
was chosen
as a were
proof-of-concept,
extremely
of the COOH
function; it was
further
reduced when
the polymer
surface
pretreated
with
its
lowconstant.
dissociation
constant.
Non-specific
interactions
reduced
due
to of
thethe
presence
lowextremely
dissociation
Non-specific
interactions
were
reducedwere
due
to
thewas
presence
COOH
1-octanol,
which
interacted
non-covalently
with P3HT
with
its
C8 alkylsurface
chain. Inwas
this pretreated
case, humanwith
of
the
COOH
function;
it
was
further
reduced
when
the
polymer
function; it was further reduced when the polymer surface was pretreated with 1-octanol, which
serumwhich
albumin
had no effect
on the transistor characteristics,
whereas
streptavidin
ledthis
to a decrease
1-octanol,
interacted
non-covalently
P3HTchain.
with In
itsthis
C8 alkyl
chain. In
human
interacted
non-covalently
with
P3HT with itswith
C8 alkyl
case, human
serum case,
albumin
had
of
the
drain
current
(Figure
11B).
serum
albumin
had
no
effect
on
the
transistor
characteristics,
whereas
streptavidin
led
to
a
decrease
no effect on the transistor characteristics, whereas streptavidin led to a decrease of the drain current
of
the drain
(Figure
11B).current (Figure 11B).
(A)
(A)
Figure 11. Cont.
Electronics 2016, 5, 9
12 of 24
Electronics 2016, 5, 9
12 of 23
Electronics 2016, 5, 9
12 of 23
(B)
Figure 11. (A) Structure of P3HT:P3HT-COOH:P3HT-biotin terpolymer. (B) Transfer curves at saturation
Figure 11. (A) Structure of P3HT:P3HT-COOH:P3HT-biotin terpolymer. (B) Transfer curves at
(B)
for P3HT:P3HT-COOH:P3HT-biotin based transistors
treated with 1-octanol before and after
saturation for P3HT:P3HT-COOH:P3HT-biotin based transistors treated with 1-octanol before and after
incubation with proteins. Reproduced from [93] with permission from The Royal Society of Chemistry.
Figurewith
11. (A)
StructureReproduced
of P3HT:P3HT-COOH:P3HT-biotin
terpolymer.
Transfer
at saturation
incubation
proteins.
from [93] with permission
from(B)
The
Royalcurves
Society
of Chemistry.
for P3HT:P3HT-COOH:P3HT-biotin based transistors treated with 1-octanol before and after
In order to investigate the transduction mechanisms of such EGOFET devices, Palazzo et al. [30]
incubation with proteins. Reproduced from [93] with permission from The Royal Society of Chemistry.
investigated
in 2014 the sensitivity
of their EGOFET
as a function
the Debye’s
length,Palazzo
the receptor
In order to investigate
the transduction
mechanisms
of suchofEGOFET
devices,
et al. [30]
charge,
and
the
distance
at
which
the
binding
event
takes
place.
For
this,
they
used
biotin/avidin
and
investigated
in 2014
the sensitivity
of their EGOFET
as a function
of the Debye’s
length, etthe
In order
to investigate
the transduction
mechanisms
of such EGOFET
devices, Palazzo
al. receptor
[30]
antigen/antibody interactions (C-reactive protein CRP and anti-CRP, respectively). The sensor was
investigated
in 2014 the
sensitivity
their EGOFET
as a function
of the
Debye’s
length,
the receptor and
charge,
and the distance
at which
the of
binding
event takes
place. For
this,
they used
biotin/avidin
shown to successfully detect binding events occurring at distances that are 30 times the Debye’s
charge, and theinteractions
distance at which
the binding
eventCRP
takesand
place.
For this, respectively).
they used biotin/avidin
and was
antigen/antibody
(C-reactive
protein
anti-CRP,
The sensor
length value from the transistor electronic channel, and even to deliver higher responses in the
antigen/antibody
interactions
(C-reactive
protein
CRP
and
anti-CRP,
respectively).
The
sensor
was
shown
to successfully
detect
binding events
occurring
distanceswas
thatnot
arecalculated,
30 times the
Debye’s
length
presence
of high salt
concentrations
(Figure
12A). Theatsensitivity
but the
limit of
shown
to
successfully
detect
binding
events
occurring
at
distances
that
are
30
times
the
Debye’s
valuedetection
from thewas
transistor
electronic
channel,
and even
higher
responses
the presence
−1 of CRP.
ca. 10 μg·mL
Transduction
of to
thedeliver
molecular
recognition
wasinexplained
by of
length value from the transistor electronic channel, and even to deliver higher responses in the
high capacitance
salt concentrations
12A). The sensitivity
wasthan
not by
calculated,
but
the limit
detection
changes at(Figure
the electrolyte/OSC
interface rather
electrostatic
effects
of the of
charges
presence of high
salt concentrations (Figure 12A). The sensitivity was not calculated, but the limit of
1 of
was ca.
10 µg¨
CRP.
Transduction
of the molecular recognition was explained by capacitance
carried
bymL
the´target
molecules
(Figure 12B).
detection was ca. 10 μg·mL−1 of CRP. Transduction of the molecular recognition was explained by
changes
at the electrolyte/OSC
interface rather
than rather
by electrostatic
effects ofeffects
the charges
carried by
capacitance
changes at the electrolyte/OSC
interface
than by electrostatic
of the charges
the target
molecules
(Figure
12B).
carried by the target molecules (Figure 12B).
(A)
(A)
Figure 12. Cont.
Electronics 2016, 5, 9
13 of 24
Electronics 2016, 5, 9
13 of 23
Electronics 2016, 5, 9
13 of 23
(B)
Figure 12. (A) Schematic view with different thicknesses of the bioreceptor layer. (B) Effect of the
Figure 12. (A) Schematic view with different thicknesses of the bioreceptor layer. (B) Effect
ionic strength on the fractional changes of the (a,b) current and (c,d) capacitance for a
of the ionic strength on the fractional changes
the (a,b) current and (c,d) capacitance for
(B) of
phospholipid/streptavidin/antibody (PL/SA/Ab)
(a,c) and PL/SA/Ab/CRP (b,d) multilayers.
a phospholipid/streptavidin/antibody
(PL/SA/Ab)
(a,c)
Reproduced from [30]. © 2014, WILEY-VCH Verlag GmbH &and
Co. PL/SA/Ab/CRP
KGaA, Weinheim. (b,d) multilayers.
Figure 12. (A) Schematic view with different thicknesses of the bioreceptor layer. (B) Effect of the
Reproduced
from [30]. © 2014, WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
ionic strength on the fractional changes of the (a,b) current and (c,d) capacitance for a
phospholipid/streptavidin/antibody (PL/SA/Ab) (a,c) and PL/SA/Ab/CRP (b,d) multilayers.
Non-Covalent
the Semiconductor
Reproduced
fromobjective
[30]. © 2014,
WILEY-VCH
Verlag
& Co. KGaA,
Still withFunctionalization
the
to of
functionalize
the GmbH
semiconductor
andWeinheim.
impede its doping by ions
5.4. Non-Covalent Functionalization of the Semiconductor
5.4.
present
theobjective
electrolyte,
et al. described
a non-covalent
approachits
using
a phospholipid
Still
withinthe
to Cotrone
functionalize
the semiconductor
and impede
doping
by ions present
5.4.
Non-Covalent
Functionalization
of the Semiconductor
bilayer
[93].
They
compared
the
electric
performances
of
the
EGOFET
including
a
phospholipid
film [93].
in the electrolyte, Cotrone et al. described a non-covalent approach using a phospholipid bilayer
to those
with
the
organic
semiconductor
directly
in
contact
with
the
gating
solution.
Impedance
Still withthe
theelectric
objective
to functionalize
the EGOFET
semiconductor
and impede
its doping film
by ions
They compared
performances
of the
including
a phospholipid
to those
spectroscopy
was
employed
to show
that
this phospholipid
bilayer
minimizes
thea penetration
of
present
in
the
electrolyte,
Cotrone
et
al.
described
a
non-covalent
approach
using
phospholipid
with the organic semiconductor directly in contact with the gating solution. Impedance spectroscopy
ions
into
the
OSC
thus
improves
the
field-effect
mode
of
operation
and
discriminates
the
bilayer [93]. They compared the electric performances of the EGOFET including a phospholipid film
was employed
to show
that
this phospholipid
bilayerorminimizes
the but
penetration
ions into the
electrochemical
one.
No
bioprobes
were immobilized
into this
this
work isof
a significant
to
those with the
organic
semiconductor
directly inoncontact
withbilayer
the gating
solution.
Impedance
OSC thus
improves
the field-effect
mode of operation and discriminates the electrochemical one.
step toward
EGOFETs
biocompatibilization.
spectroscopy
was employed
to show that this phospholipid bilayer minimizes the penetration of
No bioprobes
were
immobilized
on
oretthe
into
this biotin-labeled
bilayer
but of
this
work is and
a significant
toward
One
year
later
[64],
Magliulo
al. used
phospholipids,
indiscriminates
order tostep
form
a
ions into the OSC thus improves
field-effect
mode
operation
the
streptavidin
binding
layer
(Figure
13).
They
achieved
detection
with
a
LoD
of
10
nM
even
in
a
high
EGOFETs
biocompatibilization.
electrochemical one. No bioprobes were immobilized on or into this bilayer but this work is a significant
ionictoward
strength
solution.
They attributed
sensing
mechanism phospholipids,
to the capacitive in
effect
across
One
year
later
[64],biocompatibilization.
Magliulo
et al. the
used
biotin-labeled
order
to the
form a
step
EGOFETs
phospholipid
bilayer,
involving
the
charges
carried
by
streptavidin.
Because
the
biotinylated
One binding
year laterlayer
[64],(Figure
Magliulo13).
et al.
usedachieved
biotin-labeled
phospholipids,
in order
form
a in a
streptavidin
They
detection
with a LoD
of 10 to
nM
even
phospholipid
can easily
be(Figure
functionalized
any receptors,
this of
work
paved
the
way
for
binding
layer
13). Theywith
achieved
detection
with a LoD
10capacitive
nM
even
ineffect
a high
high streptavidin
ionic
strength
solution.
They
attributed
thevirtually
sensing
mechanism
to the
across
immunosensors,
captureThey
antibodies
usually
modified
by streptavidin
for hetergeneous
ELISA
ionic
strength solution.
attributed
the being
sensing
mechanism
to the capacitive
effect
across
the
the phospholipid
bilayer, involving
the charges
carried
by streptavidin.
Because
the
biotinylated
immunoassay.
phospholipid
bilayer,
involving
the
charges
carried
by
streptavidin.
Because
the
biotinylated
phospholipid can easily be functionalized with virtually any receptors, this work paved the way
phospholipid can easily be functionalized with virtually any receptors, this work paved the way for
for immunosensors, capture antibodies usually being modified by streptavidin for hetergeneous
immunosensors, capture antibodies usually being modified by streptavidin for hetergeneous ELISA
ELISA immunoassay.
immunoassay.
Figure 13. (a) Transfer characteristics of the biotin-functionalized phospholipid bilayer-EGOFET in
PBS, pH 7.4 (open symbols) and streptavidin (full symbols) solutions. (b,c) Diagram for the rationale
leading to the IDS current increase. Reproduced from [64]. Copyright © 2013, WILEY-VCH Verlag
GmbH & Co. KGaA, Weinheim
Figure
13. Transfer
(a) Transfer
characteristicsofofthe
thebiotin-functionalized
biotin-functionalized phospholipid
bilayer-EGOFET
in
Figure
13. (a)
characteristics
phospholipid
bilayer-EGOFET
in
PBS,
pH
7.4
(open
symbols)
and
streptavidin
(full
symbols)
solutions.
(b,c)
Diagram
for
the
rationale
PBS, pH 7.4 (open symbols) and streptavidin (full symbols) solutions. (b,c) Diagram for the rationale
leading to the IDS current increase. Reproduced from [64]. Copyright © 2013, WILEY-VCH Verlag
leading to the IDS current increase. Reproduced from [64]. Copyright © 2013, WILEY-VCH Verlag
GmbH & Co. KGaA, Weinheim
GmbH & Co. KGaA, Weinheim.
Electronics 2016, 5, 9
14 of 24
Electronics 2016, 5, 9
14 of 23
5.5. pBTTT-Based EGOFET for Protein (Streptavidin) Detection
5.5. pBTTT-Based EGOFET for Protein (Streptavidin) Detection
Mulla et al. [76] reported a pBTTT-based EGOFET sensor for streptavidin detection. pBTTT was
Mulla et al. [76] reported a pBTTT-based EGOFET sensor for streptavidin detection. pBTTT was
spin-coated on the device, then a layer of poly(acrylic acid) was spin-coated on the pBTTT layer to
spin-coated on the device, then a layer of poly(acrylic acid) was spin-coated on the pBTTT layer to
have have
carboxyl
functionalities
acting as
anchoring
sites for phospholipid
bilayers. Thebilayers.
proof-of-concept
carboxyl
functionalities
acting
as anchoring
sites for phospholipid
The
of theproof-of-concept
sensor was demonstrated
with
the
streptavidin/biotin
couple
(Figure
14).
of the sensor was demonstrated with the streptavidin/biotin couple (Figure 14).
(A)
(B)
Figure 14. (A) Schematic illustration of the fabrication process of an EGOFET biosensor: (a) after
Figure 14. (A) Schematic illustration of the fabrication process of an EGOFET biosensor: (a) after
deposition of pBTTT on the substrate; (b) poly(acrylic acid) coating spin-coated and cross-linked; (c)
deposition of pBTTT on the substrate; (b) poly(acrylic acid) coating spin-coated and cross-linked;
biotinylated phospholipids anchored on the PAA coating; and (d) EGOFET configuration
(c) biotinylated phospholipids anchored on the PAA coating; and (d) EGOFET configuration comprising
comprising a gold gate electrode and a droplet of PBS as dielectric. (B) (a) IDS–VG transfer curves in
a gold
gatepH
electrode
and
a droplet
of PBS
as dielectric.
(B) (a) IDSof
–V0.16
curvesand
in PBS,
pH 7.4
G transfer
PBS,
7.4 (line),
and
streptavidin
solutions
at concentrations
nM (circles)
1.6 μM
(line),(triangles)
and streptavidin
solutions
at concentrations
of 0.16
nM (circles)
and 1.6ofµM
in PBS;
in PBS; and
(b) relative
response of the
EGOFET
as a function
the(triangles)
streptavidin
and (b)
relative response
of thefrom
EGOFET
as permission
a function from
of the
streptavidin
concentration.
concentration.
Reproduced
[76] with
the
Royal Society
of Chemistry. Reproduced
from [76] with permission from the Royal Society of Chemistry.
5.6. Gate Functionalization
5.6. Gate Functionalization
Casalini et al. [32] reported in 2013 a P3HT-based EGOFET biosensor for dopamine detection
where a gold gate was used as the sensing area instead of the semiconductor. They modified the
Casalini
et al. [32] reported in 2013 a P3HT-based EGOFET biosensor for dopamine detection
gate/electrolyte interface with a self-assembled monolayer of cysteamine and 4-formylphenyl
where a gold gate was used as the sensing area instead of the semiconductor. They modified the
boronic acid. This modified gate enabled the selective covalent binding of dopamine that, in turn,
gate/electrolyte
interface with a self-assembled monolayer of cysteamine and 4-formylphenyl boronic
modulates both the work function of the gate electrode and the capacitance of the
acid. electrode/electrolyte
This modified gate double
enabledlayer.
the selective
covalent
binding
that, in turn,
The strong
dependence
of of
thedopamine
EGOFET transfer
curve modulates
upon
both the work function of the gate electrode and the capacitance of the electrode/electrolyte double
layer. The strong dependence of the EGOFET transfer curve upon dopamine coupling at the gate
electrode is a proof of its sensitivity to small changes in gate capacitance. For dopamine detection,
Electronics 2016, 5, 9
15 of 24
Electronics 2016, 5, 9
15 of 23
the threshold voltage shift was of ca. 50 mV for 0.1 nM dopamine. The selectivity of boronic acid for
dopamine
at by
thethe
gate
electrode
is a proof
its sensitivity
gate
dopamine
was coupling
supported
fact
that boronic
acid isofknown
to act astoa small
Lewischanges
acid forinassociation
capacitance.
For
dopamine
detection,
the
threshold
voltage
shift
was
of
ca.
50
mV
for
0.1
nM also
with vicinal glycols such as sugars or catechol, via formation of a boronate ester. This reaction
dopamine. The selectivity of boronic acid for dopamine was supported by the fact that boronic acid
works well on dopamine and is therefore specific, providing that no other vicinal diols are present in
is known to act as a Lewis acid for association with vicinal glycols such as sugars or catechol, via
the analyzed sample (this reaction has been often reported in the literature dedicated to dopamine
formation of a boronate ester. This reaction also works well on dopamine and is therefore specific,
sensing
since 2013).
providing that no other vicinal diols are present in the analyzed sample (this reaction has been often
Casalini
et al.
published
more
reported in
the[105]
literature
dedicated
to recently
dopamineanother
sensingwork
since using
2013). Au gate as sensing area, instead of
the semiconductor,
using
or a 6-aminohexanethiol
) self-assembled
6 NH2area,
Casalini et al.
[105]thiol-modified
published moreprotein
recentlyGanother
work using Au gate (HSC
as sensing
instead
monolayer
by covalent
coupling (protein
G was
here for its property
to6NH
bind
of the followed
semiconductor,
using thiol-modified
protein
G orused
a 6-aminohexanethiol
(HSC
2) IgG
self-assembled
followed
by covalent
coupling
(protein
was used here
its first
property
antibodies).
Theymonolayer
found that
the most
sensitive
EGOFET
wasG obtained
withfor
the
(affinity)
to bind IgG antibodies).
They explained
found that this
the most
sensitive
was obtained
withantibodies
the first on
immobilization
strategy. They
result
by theEGOFET
best orientation
of the
(affinity)
immobilization
strategy.
They
explained
this
result
by
the
best
orientation
of
the
antibodies
the gate surface, which they probed by force spectroscopy (Figure 15). The lowest concentration of
on the gate surface, which they probed by force spectroscopy (Figure 15). The lowest concentration
antigen
(interleukin, IL4) that was detected was ca. 5 nM. These results, and particularly the coupling
of antigen (interleukin, IL4) that was detected was ca. 5 nM. These results, and particularly the
between sensing experiments and surface characterizations, were very powerful to rationalize the
coupling between sensing experiments and surface characterizations, were very powerful to
response
of an EGOFET using antibodies grafted on the gate.
rationalize the response of an EGOFET using antibodies grafted on the gate.
(A)
(B)
Figure 15. (A) Schematic illustration of the gate-modified EGOFET, and of the AFM measurements.
Figure 15. (A) Schematic illustration of the gate-modified EGOFET, and of the AFM measurements.
(B) I-V transfer characteristics for (a) HSC6NH2- and (b) Protein G-based protocols. Normalized
(B) I-V transfer characteristics for (a) HSC6 NH2 - and (b) Protein G-based protocols. Normalized
mobility ratio (c) and threshold voltage (d) trends corresponding to the stepwise functionalization.
mobility
ratio (c) and threshold voltage (d) trends corresponding to the stepwise functionalization.
Adapted with permission from [105]. Copyright © 2015, American Chemical Society.
Adapted with permission from [105]. Copyright © 2015, American Chemical Society.
Electronics 2016, 5, 9
16 of 24
Electronics 2016, 5, 9
16 of 23
Mulla etet al.
al. [77]
[77] also
also described
described an
an EGOFET
EGOFET where
where the
the gate
gate was
was the
the sensing
They
Mulla
sensing element.
element. They
immobilized
proteins
on
the
Au
gate
through
a
self-assembled
monolayer
and
showed
that
the
immobilized proteins on the Au gate through a self-assembled monolayer and showed that the
transduction capability
device
waswas
governed
by the
of the gate/electrolyte
interface.
transduction
capability ofofthe
the
device
governed
bycapacitance
the capacitance
of the gate/electrolyte
The
authors
applied
this
architecture
to
the
detection
of
odorant
molecules
(Figure
16),
by
following
interface. The authors applied this architecture to the detection of odorant molecules (Figure 16), by
drain current
as acurrent
function
target molecule
concentration
The sensor
presented
a limit
following
drain
asofa the
function
of the target
molecule (carvone).
concentration
(carvone).
The sensor
of detection
estimated
aroundestimated
10 pM and
was enantioselective.
presented
a limit
of detection
around
10 pM and was enantioselective.
(a)
(b)
(c)
(d)
Figure 16. EGOFET where the gate is modified by a SAM of odorant binding protein (pOBP-SAM).
Figure 16. EGOFET where the gate is modified by a SAM of odorant binding protein (pOBP-SAM).
(a) Schematic structure. The OSC is pBTTT-C14. (b) pOBP protein structure and interaction with the
(a) Schematic structure. The OSC is pBTTT-C14. (b) pOBP protein structure and interaction with
odorant molecule. (c) IDS–VG transfer characteristics reported as a function to concentrations of the
the odorant molecule. (c) IDS –VG transfer characteristics reported as a function to concentrations of
odorant molecule (carvone). (d) Calibration curves for the (R)-(−)- and (S)-(+)-carvone and for a
the odorant molecule (carvone). (d) Calibration curves for the (R)-(´)- and (S)-(+)-carvone and for a
non-specific molecule. Reproduced from [77] under Creative Commons Attribution license.
non-specific molecule. Reproduced from [77] under Creative Commons Attribution license.
5.7. Other Type of Electrolyte
5.7. Other Type of Electrolyte
More sophisticated electrolytes can be used in EGOFETs in order to maximize the capacitance
More sophisticated electrolytes can be used in EGOFETs in order to maximize the capacitance
changes upon a molecular recognition event. Even if their work has not been significantly cited yet,
changes upon a molecular recognition event. Even if their work has not been significantly cited yet,
Lee et al. introduced this concept in 2013 [106]. They used bovine serum albumin (BSA) as electrolyte,
Lee et al. introduced this concept in 2013 [106]. They used bovine serum albumin (BSA) as electrolyte,
because BSA has good hydration ability, containing a high percentage of acidic and basic amino acid
because BSA has good hydration ability, containing a high percentage of acidic and basic amino acid
residues. They showed that, in dry state, BSA-gated pentacene OFETs exhibited a threshold voltage
residues. They showed that, in dry state, BSA-gated pentacene OFETs exhibited a threshold voltage of
of ca. −16 V, whereas the VTH was reduced to −0.7 V under humid conditions.
ca. ´16 V, whereas the VTH was reduced to ´0.7 V under humid conditions.
Very recently, Dumitru et al. [107] used a pH-sensitive alginate hydrogel as electrolyte (Figure
Very recently, Dumitru et al. [107] used a pH-sensitive alginate hydrogel as electrolyte
17a,b), which is biocompatible and biodegradable. To demonstrate the efficiency of such hydrogels
(Figure 17a,b), which is biocompatible and biodegradable. To demonstrate the efficiency of such
to host bioreceptor without damaging its bioactivity, glucose oxidase was entrapped into the
hydrogels to host bioreceptor without damaging its bioactivity, glucose oxidase was entrapped into
alginate in order to make a biosensor. The enzyme activity, producing an acidification of the
the alginate in order to make a biosensor. The enzyme activity, producing an acidification of the
medium through transformation of glucose into gluconic acid, modified the gel swelling (polymer
medium through transformation of glucose into gluconic acid, modified the gel swelling (polymer
chains conformational switch) that lead to a capacitance modulation and therefore a field effect
chains conformational switch) that lead to a capacitance modulation and therefore a field effect change
change (Figure 17e). The sensitivity toward glucose was not given.
(Figure 17e). The sensitivity toward glucose was not given.
Electronics 2016, 5, 9
17 of 24
Electronics 2016, 5, 9
17 of 23
(a)
(b)
Figure
Figure 17.
17. Schematic
Schematic illustration
illustration (a)
(a) and
and picture
picture (b)
(b) of
ofthe
thealginate-gated
alginate-gatedOFET;
OFET; output
output characteristics
characteristics
(c)
and
transfer
curves
(d);
and
(e)
I
DS changes versus time, for injection of water (blank) and glucose.
(c) and transfer curves (d); and (e) IDS changes versus time, for injection of water (blank) and glucose.
Reproduced
Commons Attribution
Attribution 3.0
3.0 Unported
Unported license.
license.
Reproduced from
from [107]
[107] under
under Creative
Creative Commons
5.8. Use of Nanoparticles
5.8. Use of Nanoparticles
The use of nanoparticles (NPs) has not been reported yet in EGOFET devices for biosensing.
The use of nanoparticles (NPs) has not been reported yet in EGOFET devices for biosensing.
However, NPs have been extensively reported in other type of biosensors for years, mainly because
However, NPs have been extensively reported in other type of biosensors for years, mainly because
NPs provide an extremely high surface-to-volume ratio, i.e., a high specific active area, which
NPs provide an extremely high surface-to-volume ratio, i.e., a high specific active area, which makes
makes bioprobes more accessible than on a planar surface. In addition, because EGOFETs are
bioprobes more accessible than on a planar surface. In addition, because EGOFETs are sensitive to
sensitive to capacitance changes (a typical surface effect), NPs should be extremely pertinent in
capacitance changes (a typical surface effect), NPs should be extremely pertinent in EGOFETs. As
EGOFETs. As an example of what could be done, Poghossian et al., not in an organic-based but in a
an example of what could be done, Poghossian et al., not in an organic-based but in a silicon-based
silicon-based device, reported a gating capacitive field-effect sensor [108] for detection of charged
device, reported a gating capacitive field-effect sensor [108] for detection of charged molecules using
molecules using gold NPs to increase the capacitive effect (Figure 18). Bao’s group also reported
gold NPs to increase the capacitive effect (Figure 18). Bao’s group also reported biodetection using
biodetection using a classical bottom-gate OFET decorated with gold nanoparticles, which were
used to vary spacing between receptors [109].
Electronics 2016, 5, 9
18 of 24
a classical bottom-gate OFET decorated with gold nanoparticles, which were used to vary spacing
between
receptors
[109].
Electronics 2016,
5, 9
18 of 23
Figure 18.
18. Schematic
of (a)
(a) aa capacitive
capacitive Al/p-Si/SiO
Al/p-Si/SiO2 sensor
after silanization; (b) deposition of
Figure
Schematic of
2 sensor after silanization; (b) deposition of
negatively
charged
citrate-capped
AuNPs
(b);
and
(c)
adsorption
of positively
positively charged
charged molecules
molecules on
on
negatively charged citrate-capped AuNPs (b); and (c) adsorption of
the
AuNPs.
(d)
Capacitance
vs.
gate
voltage
curves.
Reproduced
from
[108]
with
permission
from
the AuNPs. (d) Capacitance vs. gate voltage curves. Reproduced from [108] with permission from the
the Royal
Society
of Chemistry.
Royal
Society
of Chemistry.
6. Conclusions and Outlooks
6. Conclusions and Outlooks
In this review, we presented recent advances in electrolyte-gated field-effect transistors applied
In this review, we presented recent advances in electrolyte-gated field-effect transistors
for biosensors. EGOFETs rely on the accumulation of charges at the gate/dielectric and
applied for biosensors. EGOFETs rely on the accumulation of charges at the gate/dielectric and
dielectric/semiconductor interfaces. The detection principles in EGOFETs are based on changes of
dielectric/semiconductor interfaces. The detection principles in EGOFETs are based on changes of these
these surface capacitances induced by the biological recognition between probe and target
surface capacitances induced by the biological recognition between probe and target molecules. The
molecules. The electrolyte, which replaces the classical dielectric in EGOFET, fits well with biological
electrolyte, which replaces the classical dielectric in EGOFET, fits well with biological processes (which
processes (which generally take place in water), whereas it is less straightforward to apply regular
generally take place in water), whereas it is less straightforward to apply regular dielectric-based OFETs
dielectric-based OFETs for biosensing. There is still a lot of work to do to improve EGOFET-based
for biosensing. There is still a lot of work to do to improve EGOFET-based biosensor’s performances.
biosensor’s performances. The most challenging is certainly the stability of the semiconductors in
The most challenging is certainly the stability of the semiconductors in water or neutral buffers, the
water or neutral buffers, the immobilization of the bioprobes whether onto the gate or the
immobilization of the bioprobes whether onto the gate or the semiconductor surface, and a better
semiconductor surface, and a better understanding of the operating principles for the design of more
understanding of the operating principles for the design of more efficient transduction architectures.
efficient transduction architectures. Concerning OSC, only few were used until now (P3HT, pBTTT,
Concerning OSC, only few were used until now (P3HT, pBTTT, α-sexithiophene, and pentacene)
α-sexithiophene, and pentacene) and many others deserve to be investigated. Concerning
and many others deserve to be investigated. Concerning immobilization, different methods were
immobilization, different methods were used to immobilize the biological probes on the sensing
used to immobilize the biological probes on the sensing parts of EGOFETs, whether by covalent or
parts of EGOFETs, whether by covalent or non-covalent methods. Covalent bindings look to be
non-covalent methods. Covalent bindings look to be more efficient on the gate, whereas non-covalent
more efficient on the gate, whereas non-covalent immobilizations are more convenient on the
immobilizations are more convenient on the semiconductors because it avoids tedious chemical
semiconductors because it avoids tedious chemical derivatization, which generally impedes the
derivatization, which generally impedes the electrical performances of the OSC. Most biorecognition
electrical performances of the OSC. Most biorecognition processes reported in the literature concern
processes reported in the literature concern DNA hybridization, specific chemical couplings, DNA or
DNA hybridization, specific chemical couplings, DNA or peptide aptamers, antibody–antigen
peptide aptamers, antibody–antigen recognitions or other protein–protein interactions. Most of these
recognitions or other protein–protein interactions. Most of these methods involve capture of a free
methods involve capture of a free target in electrolyte onto a biological probe immobilized on a surface.
target in electrolyte onto a biological probe immobilized on a surface. However, an opposite way of
However, an opposite way of thinking, inspired from competitive immunoassays, is also a promising
thinking, inspired from competitive immunoassays, is also a promising approach, worth trying. At
approach, worth trying. At last, nanostructuration of gates or semiconductors should be investigated.
last, nanostructuration of gates or semiconductors should be investigated.
Acknowledgments:
D.W. thanks
Doctorate
School
ED388 for a Ph.D. grant.
Acknowledgments: Acknowledgments:
D.W. thanks the Doctorate
Schoolthe
ED388
for a Ph.D.
grant.
Author Contributions: Author Contributions: The authors contributed equally to the writing of the manuscript.
Author Contributions: The authors contributed equally to the writing of the manuscript.
Conflicts of Interest: Conflicts of Interest: The authors declare no conflict of interest.
Conflicts of Interest: The authors declare no conflict of interest.
References
1.
2.
3.
Wilkinson, A.; McNaught, A.D. IUPAC Compendium of Chemical Terminology, (the “Gold Book”); Blackwell
Scientific Publications: Oxford, UK, 1997.
Nic, M.; Jirat, J.; Kosata, B. Compendium of Chemical Terminology; Blackwell Scientific Publications: Oxford,
UK, 1997. Aviliable online: http://goldbook.iupac.org (accessed on 23 Feburary 2016)
Thévenot, D.R.; Toth, K.; Durst, R.A.; Wilson, G.S. Electrochemical biosensors: Recommended definitions
and classification. Biosens. Bioelectron. 2001, 16, 121–131.
Electronics 2016, 5, 9
19 of 24
References
1.
2.
3.
4.
5.
6.
7.
8.
9.
10.
11.
12.
13.
14.
15.
16.
17.
18.
19.
20.
21.
22.
Wilkinson, A.; McNaught, A.D. IUPAC Compendium of Chemical Terminology, (the “Gold Book”); Blackwell
Scientific Publications: Oxford, UK, 1997.
Nic, M.; Jirat, J.; Kosata, B. Compendium of Chemical Terminology; Blackwell Scientific Publications: Oxford,
UK, 1997; Available online: http://goldbook.iupac.org (accessed on 23 Feburary 2016).
Thévenot, D.R.; Toth, K.; Durst, R.A.; Wilson, G.S. Electrochemical biosensors: Recommended definitions
and classification. Biosens. Bioelectron. 2001, 16, 121–131. [CrossRef]
Clark, L.C.; Lyons, C. Electrode systems for continuous monitoring in cardiovascular surgery. Ann. N. Y.
Acad. Sci. 1962, 102, 29–45. [CrossRef] [PubMed]
Amos, S.W.; James, M. Principles of Transistor Circuits; Butterworths: London, UK, 1984.
Strakosas, X.; Bongo, M.; Owens, R.M. The organic electrochemical transistor for biological applications.
J. Appl. Polym. Sci. 2015, 132. [CrossRef]
Sarkar, D.; Banerjee, K. Proposal for tunnel-field-effect-transistor as ultra-sensitive and label-free biosensors.
Appl. Phys. Lett. 2012, 100, 143108. [CrossRef]
Kergoat, L.; Piro, B.; Berggren, M.; Horowitz, G.; Pham, M.-C. Advances in organic transistor-based
biosensors: From organic electrochemical transistors to electrolyte-gated organic field-effect transistors. Anal.
Bioanal. Chem. 2012, 402, 1813–1826. [CrossRef] [PubMed]
Tsumura, A.; Koezuka, H.; Ando, T. Macromolecular electronic device: Field-effect transistor with a
polythiophene thin film. Appl. Phys. Lett. 1986, 49, 1210–1212. [CrossRef]
Kergoat, L.; Herlogsson, L.; Braga, D.; Piro, B.; Pham, M.C.; Crispin, X.; Berggren, M.; Horowitz, G. A
water-gate organic field-effect transistor. Adv. Mater. 2010, 22, 2565–2569. [CrossRef] [PubMed]
Buth, F.; Donner, A.; Sachsenhauser, M.; Stutzmann, M.; Garrido, J.A. Biofunctional electrolyte-gated organic
field-effect transistors. Adv. Mater. 2012, 24, 4511–4517. [CrossRef] [PubMed]
Dhoot, A.S.; Yuen, J.D.; Heeney, M.; McCulloch, I.; Moses, D.; Heeger, A.J. Beyond the metal-insulator
transition in polymer electrolyte gated polymer field-effect transistors. Proc. Natl. Acad. Sci. USA 2006, 103,
11834–11837. [CrossRef] [PubMed]
Tarabella, G.; Mohammadi, F.M.; Coppedè, N.; Barbero, F.; Iannotta, S.; Santato, C.; Cicoira, F. New
opportunities for organic electronics and bioelectronics: Ions in action. Chem. Sci. 2013, 4, 1395–1409.
[CrossRef]
Kim, S.H.; Hong, K.; Xie, W.; Lee, K.H.; Zhang, S.; Lodge, T.P.; Frisbie, C.D. Electrolyte-gated transistors for
organic and printed electronics. Adv. Mater. 2013, 25, 1822–1846. [CrossRef] [PubMed]
Yuen, J.D.; Dhoot, A.S.; Namdas, E.B.; Coates, N.E.; Heeney, M.; McCulloch, I.; Moses, D.; Heeger, A.J.
Electrochemical doping in electrolyte-gated polymer transistors. J. Am. Chem. Soc. 2007, 129, 14367–14371.
[CrossRef] [PubMed]
Lee, J.; Kaake, L.G.; Cho, J.H.; Zhu, X.-Y.; Lodge, T.P.; Frisbie, C.D. Ion gel-gated polymer thin-film transistors:
Operating mechanism and characterization of gate dielectric capacitance, switching speed, and stability.
J. Phys. Chem. C 2009, 113, 8972–8981. [CrossRef]
Stern, E.; Klemic, J.F.; Routenberg, D.A.; Wyrembak, P.N.; Turner-Evans, D.B.; Hamilton, A.D.; LaVan, D.A.;
Fahmy, T.M.; Reed, M.A. Label-free immunodetection with cmos-compatible semiconducting nanowires.
Nature 2007, 445, 519–522. [CrossRef] [PubMed]
Stern, E.; Wagner, R.; Sigworth, F.J.; Breaker, R.; Fahmy, T.M.; Reed, M.A. Importance of the debye screening
length on nanowire field effect transistor sensors. Nano Lett. 2007, 7, 3405–3409. [CrossRef] [PubMed]
Liao, C.; Yan, F. Organic semiconductors in organic thin-film transistor-based chemical and biological sensors.
Polym. Rev. 2013, 53, 352–406. [CrossRef]
Said, E.; Crispin, X.; Herlogsson, L.; Elhag, S.; Robinson, N.D.; Berggren, M. Polymer field-effect transistor
gated via a poly (styrenesulfonic acid) thin film. Appl. Phys. Lett. 2006, 89, 143507. [CrossRef]
Herlogsson, L.; Crispin, X.; Robinson, N.D.; Sandberg, M.; Hagel, O.J.; Gustafsson, G.; Berggren, M.
Low-voltage polymer field-effect transistors gated via a proton conductor. Adv. Mater. 2007, 19, 97–101.
[CrossRef]
Said, E.; Larsson, O.; Berggren, M.; Crispin, X. Effects of the ionic currents in electrolyte-gated organic
field-effect transistors. Adv. Funct. Mater. 2008, 18, 3529–3536. [CrossRef]
Electronics 2016, 5, 9
23.
24.
25.
26.
27.
28.
29.
30.
31.
32.
33.
34.
35.
36.
37.
38.
39.
40.
41.
42.
43.
44.
45.
20 of 24
Ono, S.; Miwa, K.; Seki, S.; Takeya, J. A comparative study of organic single-crystal transistors gated with
various ionic-liquid electrolytes. Appl. Phys. Lett. 2009, 94, 063301. [CrossRef]
Xia, Y.; Cho, J.H.; Lee, J.; Ruden, P.P.; Frisbie, C.D. Comparison of the mobility-carrier density relation in
polymer and single-crystal organic transistors employing vacuum and liquid gate dielectrics. Adv. Mater.
2009, 21, 2174–2179. [CrossRef]
Hamedi, M.; Herlogsson, L.; Crispin, X.; Marcilla, R.; Berggren, M.; Inganäs, O. Fiber-embedded
electrolyte-gated field-effect transistors for e-textiles. Adv. Mater. 2009, 21, 573–577. [CrossRef] [PubMed]
Lee, J.; Panzer, M.J.; He, Y.; Lodge, T.P.; Frisbie, C.D. Ion gel gated polymer thin-film transistors. J. Am. Chem.
Soc. 2007, 129, 4532–4533. [CrossRef] [PubMed]
Cho, J.H.; Lee, J.; Xia, Y.; Kim, B.; He, Y.; Renn, M.J.; Lodge, T.P.; Frisbie, C.D. Printable ion-gel gate dielectrics
for low-voltage polymer thin-film transistors on plastic. Nat. Mater. 2008, 7, 900–906. [CrossRef] [PubMed]
Cho, J.H.; Lee, J.; He, Y.; Kim, B.; Lodge, T.P.; Frisbie, C.D. High-capacitance ion gel gate dielectrics with
faster polarization response times for organic thin film transistors. Adv. Mater. 2008, 20, 686–690. [CrossRef]
Facchetti, A. Dielectric materials: Gels excel. Nat. Mater. 2008, 7, 839–840. [CrossRef] [PubMed]
Palazzo, G.; De Tullio, D.; Magliulo, M.; Mallardi, A.; Intranuovo, F.; Mulla, M.Y.; Favia, P.;
Vikholm-Lundin, I.; Torsi, L. Detection beyond debye’s length with an electrolyte-gated organic field-effect
transistor. Adv. Mater. 2014, 27, 911–916. [CrossRef] [PubMed]
Belkhir, A. Contribution à la Modélisation des Transistors Organiques. Ph.D. Thesis, The University of Reims
Champagne-Ardenne, Reims, France, 2009.
Casalini, S.; Leonardi, F.; Cramer, T.; Biscarini, F. Organic field-effect transistor for label-free dopamine
sensing. Org. Electron. 2013, 14, 156–163. [CrossRef]
Cai, B.; Wang, S.; Huang, L.; Ning, Y.; Zhang, Z.; Zhang, G.-J. Ultrasensitive label-free detection of PNA-DNA
hybridization by reduced graphene oxide field-effect transistor biosensor. ACS Nano 2014, 8, 2632–2638.
[CrossRef] [PubMed]
Vasu, K.; Chakraborty, B.; Sampath, S.; Sood, A. Probing top-gated field effect transistor of reduced graphene
oxide monolayer made by dielectrophoresis. Solid State Commun. 2010, 150, 1295–1298. [CrossRef]
Liao, R.; Tang, Z.; Lei, Y.; Guo, B. Polyphenol-reduced graphene oxide: Mechanism and derivatization.
J. Phys. Chem. C 2011, 115, 20740–20746. [CrossRef]
Liu, F.; Kim, Y.H.; Cheon, D.S.; Seo, T.S. Micropatterned reduced graphene oxide based field-effect transistor
for real-time virus detection. Sens. Actuators B Chem. 2013, 186, 252–257. [CrossRef]
Zhao, Y.; Wei, Q.; Xu, C.; Li, H.; Wu, D.; Cai, Y.; Mao, K.; Cui, Z.; Du, B. Label-free electrochemical
immunosensor for sensitive detection of kanamycin. Sens. Actuators B Chem. 2011, 155, 618–625. [CrossRef]
Chen, T.-Y.; Loan, P.T.K.; Hsu, C.-L.; Lee, Y.-H.; Wang, J.T.-W.; Wei, K.-H.; Lin, C.-T.; Li, L.-J. Label-free
detection of DNA hybridization using transistors based on cvd grown graphene. Biosens. Bioelectron. 2013,
41, 103–109. [CrossRef] [PubMed]
Park, J.W.; Lee, C.; Jang, J. High-performance field-effect transistor-type glucose biosensor based on
nanohybrids of carboxylated polypyrrole nanotube wrapped graphene sheet transducer. Sens. Actuators B
Chem. 2015, 208, 532–537. [CrossRef]
Williams, G.; Kamat, P.V. Graphene-semiconductor nanocomposites: Excited-state interactions between zno
nanoparticles and graphene oxide†. Langmuir 2009, 25, 13869–13873. [CrossRef] [PubMed]
Feng, L.; Chen, Y.; Ren, J.; Qu, X. A graphene functionalized electrochemical aptasensor for selective label-free
detection of cancer cells. Biomaterials 2011, 32, 2930–2937. [CrossRef] [PubMed]
Ohno, Y.; Maehashi, K.; Yamashiro, Y.; Matsumoto, K. Electrolyte-gated graphene field-effect transistors for
detecting ph and protein adsorption. Nano Lett. 2009, 9, 3318–3322. [CrossRef] [PubMed]
Tran, H.; Piro, B.; Reisberg, S.; Nguyen, L.H.; Nguyen, T.D.; Duc, H.; Pham, M. An electrochemical elisa-like
immunosensor for mirnas detection based on screen-printed gold electrodes modified with reduced graphene
oxide and carbon nanotubes. Biosens. Bioelectron. 2014, 62, 25–30. [CrossRef] [PubMed]
Dong, X.; Shi, Y.; Huang, W.; Chen, P.; Li, L.J. Electrical detection of DNA hybridization with single-base
specificity using transistors based on cvd-grown graphene sheets. Adv. Mater. 2010, 22, 1649–1653. [CrossRef]
[PubMed]
Ge, S.; Lan, F.; Yu, F.; Yu, J. Applications of graphene and related nanomaterials in analytical chemistry. New
J. Chem. 2015, 39, 2380–2395. [CrossRef]
Electronics 2016, 5, 9
46.
47.
48.
49.
50.
51.
52.
53.
54.
55.
56.
57.
58.
59.
60.
61.
62.
63.
64.
65.
66.
21 of 24
Tran, H.; Piro, B.; Reisberg, S.; Duc, H.; Pham, M. Antibodies directed to rna/DNA hybrids: An
electrochemical immunosensor for micrornas detection using graphene-composite electrodes. Anal. Chem.
2013, 85, 8469–8474. [CrossRef] [PubMed]
Yan, F.; Zhang, M.; Li, J. Solution-gated graphene transistors for chemical and biological sensors. Adv. Healthc.
Mater. 2014, 3, 313–331. [CrossRef] [PubMed]
Sarkar, D.; Liu, W.; Xie, X.; Anselmo, A.C.; Mitragotri, S.; Banerjee, K. MoS2 Field-Effect Transistor for
Next-Generation Label-Free Biosensors. ACS Nano 2014, 8, 3992–4003. [CrossRef] [PubMed]
Kergoat, L.; Herlogsson, L.; Piro, B.; Pham, M.C.; Horowitz, G.; Crispin, X.; Berggren, M. Tuning the threshold
voltage in electrolyte-gated organic field-effect transistors. Proc. Natl. Acad. Sci. USA 2012, 109, 8394–8399.
[CrossRef] [PubMed]
Fabiano, S.; Braun, S.; Fahlman, M.; Crispin, X.; Berggren, M. Effect of gate electrode work-function on source
charge injection in electrolyte-gated organic field-effect transistors. Adv. Funct. Mater. 2014, 24, 695–700.
[CrossRef]
Demelas, M.; Lai, S.; Spanu, A.; Martinoia, S.; Cosseddu, P.; Barbaro, M.; Bonfiglio, A. Charge sensing by
organic charge-modulated field effect transistors: Application to the detection of bio-related effects. J. Mater.
Chem. B 2013, 1, 3811–3819. [CrossRef]
Myers, J.D.; Xue, J. Organic semiconductors and their applications in photovoltaic devices. Polym. Rev. 2012,
52, 1–37. [CrossRef]
Alberga, D.; Mangiatordi, G.F.; Torsi, L.; Lattanzi, G. Effects of annealing and residual solvents on amorphous
p3ht and pbttt films. J. Phys. Chem. C 2014, 118, 8641–8655. [CrossRef]
Sirringhaus, H.; Brown, P.; Friend, R.; Nielsen, M.M.; Bechgaard, K.; Langeveld-Voss, B.; Spiering, A.;
Janssen, R.A.; Meijer, E.; Herwig, P. Two-dimensional charge transport in self-organized, high-mobility
conjugated polymers. Nature 1999, 401, 685–688. [CrossRef]
Derby, B. Inkjet printing of functional and structural materials: Fluid property requirements, feature stability,
and resolution. Annu. Rev. Mater. Res. 2010, 40, 395–414. [CrossRef]
De Gans, B.J.; Duineveld, P.C.; Schubert, U.S. Inkjet printing of polymers: State of the art and future
developments. Adv. Mater. 2004, 16, 203–213. [CrossRef]
Liu, C.; Xu, Y.; Liu, Z.; Tsao, H.N.; Müllen, K.; Minari, T.; Noh, Y.-Y.; Sirringhaus, H. Improving
solution-processed n-type organic field-effect transistors by transfer-printed metal/semiconductor and
semiconductor/semiconductor heterojunctions. Org. Electron. 2014, 15, 1884–1889. [CrossRef]
Noh, Y.-Y.; Zhao, N.; Caironi, M.; Sirringhaus, H. Downscaling of self-aligned, all-printed polymer thin-film
transistors. Nat. Nanotechnol. 2007, 2, 784–789. [CrossRef] [PubMed]
Sinno, H.; Nguyen, H.T.; Hägerström, A.; Fahlman, M.; Lindell, L.; Coulembier, O.; Dubois, P.; Crispin, X.;
Engquist, I.; Berggren, M. Amphiphilic semiconducting copolymer as compatibility layer for printing
polyelectrolyte-gated ofets. Org. Electron. 2013, 14, 790–796. [CrossRef]
Wang, X.; Nilsson, D.; Norberg, P. Printable microfluidic systems using pressure sensitive adhesive material
for biosensing devices. Biochim. Biophys. Acta 2013, 1830, 4398–4401. [CrossRef] [PubMed]
Thiemann, S.; Sachnov, S.; Gruber, M.; Gannott, F.; Spallek, S.; Schweiger, M.; Krückel, J.; Kaschta, J.;
Spiecker, E.; Wasserscheid, P. Spray-coatable ionogels based on silane-ionic liquids for low voltage, flexible,
electrolyte-gated organic transistors. J. Mater. Chem. C 2014, 2, 2423–2430. [CrossRef]
Buth, F.; Kumar, D.; Stutzmann, M.; Garrido, J. Electrolyte-gated organic field-effect transistors for sensing
applications. Appl. Phys. Lett. 2011, 98, 153302. [CrossRef]
Scarpa, G.; Idzko, A.; Götz, S.; Neumaier, T.; Thalhammer, S. Biocompatibility studies of solution-processable
organic thin-film transistors for sensing applications. In Proceedings of the IEEE International Conference on
Nano/Molecular Medicine and Engineering (NANOMED), Tainan, Taiwan, 18–21 October 2009; pp. 265–268.
Magliulo, M.; Mallardi, A.; Mulla, M.Y.; Cotrone, S.; Pistillo, B.R.; Favia, P.; Vikholm-Lundin, I.;
Palazzo, G.; Torsi, L. Electrolyte-gated organic field-effect transistor sensors based on supported biotinylated
phospholipid bilayer. Adv. Mater. 2013, 25, 2090–2094. [CrossRef] [PubMed]
Sokolov, A.N.; Roberts, M.E.; Bao, Z. Fabrication of low-cost electronic biosensors. Mater. Today 2009, 12,
12–20. [CrossRef]
Angione, M.D.; Cotrone, S.; Magliulo, M.; Mallardi, A.; Altamura, D.; Giannini, C.; Cioffi, N.; Sabbatini, L.;
Fratini, E.; Baglioni, P. Interfacial electronic effects in functional biolayers integrated into organic field-effect
transistors. Proc. Natl. Acad. Sci. USA 2012, 109, 6429–6434. [CrossRef] [PubMed]
Electronics 2016, 5, 9
67.
68.
69.
70.
71.
72.
73.
74.
75.
76.
77.
78.
79.
80.
81.
82.
83.
84.
85.
86.
22 of 24
Yan, F.; Mok, S.M.; Yu, J.; Chan, H.L.; Yang, M. Label-free DNA sensor based on organic thin film transistors.
Biosens. Bioelectron. 2009, 24, 1241–1245. [CrossRef] [PubMed]
Maddalena, F.; Kuiper, M.J.; Poolman, B.; Brouwer, F.; Hummelen, J.C.; de Leeuw, D.M.; De Boer, B.;
Blom, P.W. Organic field-effect transistor-based biosensors functionalized with protein receptors. J. Appl.
Phys. 2010, 108, 124501. [CrossRef]
Roberts, M.E.; Mannsfeld, S.C.; Queraltó, N.; Reese, C.; Locklin, J.; Knoll, W.; Bao, Z. Water-stable organic
transistors and their application in chemical and biological sensors. Proc. Natl. Acad. Sci. USA 2008, 105,
12134–12139. [CrossRef] [PubMed]
Kergoat, L.; Piro, B.; Berggren, M.; Pham, M.-C.; Yassar, A.; Horowitz, G. DNA detection with a water-gated
organic field-effect transistor. Org. Electron. 2012, 13, 1–6. [CrossRef]
Magliulo, M.; Pistillo, B.R.; Mulla, M.Y.; Cotrone, S.; Ditaranto, N.; Cioffi, N.; Favia, P.; Torsi, L. PE-CVD
of hydrophilic-COOH functionalized coatings on electrolyte gated field-effect transistor electronic layers.
Plasma Process. Polym. 2013, 10, 102–109. [CrossRef]
Cotrone, S.; Ambrico, M.; Toss, H.; Angione, M.D.; Magliulo, M.; Mallardi, A.; Berggren, M.; Palazzo, G.;
Horowitz, G.; Ligonzo, T. Phospholipid film in electrolyte-gated organic field-effect transistors. Org. Electron.
2012, 13, 638–644. [CrossRef]
McCulloch, I.; Heeney, M.; Chabinyc, M.L.; DeLongchamp, D.; Kline, R.J.; Colle, M.; Duffy, W.; Fischer, D.;
Gundlach, D.; Hamadani, B.; et al. Semiconducting Thienothiophene Copolymers: Design, Synthesis,
Morphology, and Performance in Thin-Film Organic Transistors. Adv. Mater. 2009, 21, 1091–1109. [CrossRef]
Minamiki, T.; Minami, T.; Kurita, R.; Niwa, O.; Wakida, S.-I.; Fukuda, K.; Kumaki, D.; Tokito, S. Accurate and
reproducible detection of proteins in water using an extended-gate type organic transistor biosensor. Appl.
Phys. Lett. 2014, 104, 243703. [CrossRef]
Dumitru, L.M.; Manoli, K.; Magliulo, M.; Palazzo, G.; Torsi, L. Low-voltage solid electrolyte-gated ofets for
gas sensing applications. Microelectron. J. 2014, 45, 1679–1683. [CrossRef]
Mulla, M.; Seshadri, P.; Torsi, L.; Manoli, K.; Mallardi, A.; Ditaranto, N.; Santacroce, M.; Di Franco, C.;
Scamarcio, G.; Magliulo, M. Uv crosslinked poly (acrylic acid): A simple method to bio-functionalize
electrolyte-gated ofet biosensors. J. Mater. Chem. B 2015, 3, 5049–5057. [CrossRef]
Mulla, M.Y.; Tuccori, E.; Magliulo, M.; Lattanzi, G.; Palazzo, G.; Persaud, K.; Torsi, L. Capacitance-modulated
transistor detects odorant binding protein chiral interactions. Nat. Commun. 2015, 6. [CrossRef] [PubMed]
Kline, R.J.; McGehee, M.D.; Kadnikova, E.N.; Liu, J.; Frechet, J.M. Controlling the field-effect mobility of
regioregular polythiophene by changing the molecular weight. Adv. Mater. 2003, 15, 1519–1522. [CrossRef]
Chang, J.-F.; Sun, B.; Breiby, D.W.; Nielsen, M.M.; Sölling, T.I.; Giles, M.; McCulloch, I.; Sirringhaus, H.
Enhanced mobility of poly (3-hexylthiophene) transistors by spin-coating from high-boiling-point solvents.
Chem. Mater. 2004, 16, 4772–4776. [CrossRef]
Heffner, G.W.; Pearson, D.S. Molecular characterization of poly (3-hexylthiophene). Macromolecules 1991, 24,
6295–6299. [CrossRef]
Bao, Z.; Dodabalapur, A.; Lovinger, A.J. Soluble and processable regioregular poly (3-hexylthiophene)
for thin film field-effect transistor applications with high mobility. Appl. Phys. Lett. 1996, 69, 4108–4110.
[CrossRef]
Sirringhaus, H.; Tessler, N.; Friend, R.H. Integrated optoelectronic devices based on conjugated polymers.
Science 1998, 280, 1741–1744. [CrossRef] [PubMed]
Hugger, S.; Thomann, R.; Heinzel, T.; Thurn-Albrecht, T. Semicrystalline morphology in thin films of poly
(3-hexylthiophene). Colloid Polym. Sci. 2004, 282, 932–938.
Cho, S.; Lee, K.; Yuen, J.; Wang, G.; Moses, D.; Heeger, A.J.; Surin, M.; Lazzaroni, R. Thermal
annealing-induced enhancement of the field-effect mobility of regioregular poly (3-hexylthiophene) films.
J. Appl. Phys. 2006, 100, 114503. [CrossRef]
Gurau, M.C.; Delongchamp, D.M.; Vogel, B.M.; Lin, E.K.; Fischer, D.A.; Sambasivan, S.; Richter, L.J.
Measuring molecular order in poly (3-alkylthiophene) thin films with polarizing spectroscopies. Langmuir
2007, 23, 834–842. [CrossRef] [PubMed]
Alexiadis, O.; Mavrantzas, V.G. All-atom molecular dynamics simulation of temperature effects on the
structural, thermodynamic, and packing properties of the pure amorphous and pure crystalline phases of
regioregular p3ht. Macromolecules 2013, 46, 2450–2467. [CrossRef]
Electronics 2016, 5, 9
87.
88.
89.
90.
91.
92.
93.
94.
95.
96.
97.
98.
99.
100.
101.
102.
103.
104.
105.
106.
107.
23 of 24
Mårdalen, J.; Samuelsen, E.J.; Gautun, O.R.; Carlsen, P.H. Chain configuration of poly (3-hexylthiophene) as
revealed by detailed x-ray diffraction studies. Solid State Commun. 1991, 77, 337–339. [CrossRef]
Prosa, T.; Winokur, M.; Moulton, J.; Smith, P.; Heeger, A. X-ray structural studies of poly (3-alkylthiophenes):
An example of an inverse comb. Macromolecules 1992, 25, 4364–4372. [CrossRef]
Marchant, S.; Foot, P. Annealing behaviour of conductive poly (3-hexylthiophene) films. Polymer 1997, 38,
1749–1751. [CrossRef]
Favia, P.; Sardella, E.; Gristina, R.; d’Agostino, R. Novel plasma processes for biomaterials: Micro-scale
patterning of biomedical polymers. Surf. Coat. Technol. 2003, 169, 707–711. [CrossRef]
Kergoat, L.; Battaglini, N.; Miozzo, L.; Piro, B.; Pham, M.-C.; Yassar, A.; Horowitz, G. Use of poly
(3-hexylthiophene)/poly (methyl methacrylate)(p3ht/pmma) blends to improve the performance of
water-gated organic field-effect transistors. Org. Electron. 2011, 12, 1253–1257. [CrossRef]
Toss, H.; Suspene, C.; Piro, B.; Yassar, A.; Crispin, X.; Kergoat, L.; Pham, M.-C.; Berggren, M. On the mode
of operation in electrolyte-gated thin film transistors based on different substituted polythiophenes. Org.
Electron. 2014, 15, 2420–2427. [CrossRef]
Suspène, C.; Piro, B.; Reisberg, S.; Pham, M.-C.; Toss, H.; Berggren, M.; Yassar, A.; Horowitz, G.
Copolythiophene-based water-gated organic field-effect transistors for biosensing. J. Mater. Chem. B
2013, 1, 2090–2097. [CrossRef]
Chabinyc, M.L.; Endicott, F.; Vogt, B.D.; DeLongchamp, D.M.; Lin, E.K.; Wu, Y.; Liu, P.; Ong, B.S. Effects
of humidity on unencapsulated poly (thiophene) thin-film transistors. Appl. Phys. Lett. 2006, 88, 113514.
[CrossRef]
Hoshino, S.; Yoshida, M.; Uemura, S.; Kodzasa, T.; Takada, N.; Kamata, T.; Yase, K. Influence of moisture
on device characteristics of polythiophene-based field-effect transistors. J. Appl. Phys. 2004, 95, 5088–5093.
[CrossRef]
Caronna, T.; Forte, M.; Catellani, M.; Meille, S.V. Photodegradation and photostabilization studies of poly
(3-butylthiophene) in the solid state. Chem. Mater. 1997, 9, 991–995. [CrossRef]
Manoli, K.; Dumitru, L.M.; Mulla, M.Y.; Magliulo, M.; Franco, C.D.; Santacroce, M.V.; Scamarcio, G.; Torsi, L.
A comparative study of the gas sensing behavior in p3ht-and pbttt-based otfts: The influence of film
morphology and contact electrode position. Sensors 2014, 14, 16869–16880. [CrossRef] [PubMed]
Do, K.; Huang, D.M.; Faller, R.; Moulé, A.J. A comparative md study of the local structure of polymer
semiconductors p3ht and pbttt. Phys. Chem. Chem. Phys. 2010, 12, 14735–14739. [CrossRef] [PubMed]
Porrazzo, R.; Bellani, S.; Luzio, A.; Lanzarini, E.; Caironi, M.; Antognazza, M.R. Improving mobility and
electrochemical stability of a water-gated polymer field-effect transistor. Org. Electron. 2014, 15, 2126–2134.
[CrossRef]
Al Naim, A.F.; Grell, M. Organic solvents as gate media for thin-film transistors. J. Appl. Phys. 2012, 112,
114502. [CrossRef]
Cho, E.; Risko, C.; Kim, D.; Gysel, R.; Cates Miller, N.; Breiby, D.W.; McGehee, M.D.; Toney, M.F.; Kline, R.J.;
Bredas, J.-L. Three-dimensional packing structure and electronic properties of biaxially oriented poly (2, 5-bis
(3-alkylthiophene-2-yl) thieno [3, 2-b] thiophene) films. J. Am. Chem. Soc. 2012, 134, 6177–6190. [CrossRef]
[PubMed]
McCulloch, I.; Heeney, M.; Bailey, C.; Genevicius, K.; MacDonald, I.; Shkunov, M.; Sparrowe, D.; Tierney, S.;
Wagner, R.; Zhang, W. Liquid-crystalline semiconducting polymers with high charge-carrier mobility. Nat.
Mater. 2006, 5, 328–333. [CrossRef] [PubMed]
Tsao, H.N.; Müllen, K. Improving polymer transistor performance via morphology control. Chem. Soc. Rev.
2010, 39, 2372–2386. [CrossRef] [PubMed]
Schmoltner, K.; Kofler, J.; Klug, A.; List-Kratochvil, E. Electrolyte-gated organic field-effect transistors for
sensing in aqueous media. SPIE Proc. 2013. [CrossRef]
Casalini, S.; Dumitru, A.C.; Leonardi, F.; Bortolotti, C.A.; Herruzo, E.T.; Campana, A.; de Oliveira, R.F.;
Cramer, T.; Garcia, R.; Biscarini, F. Multiscale sensing of antibody-antigen interactions by organic transistors
and single-molecule force spectroscopy. ACS Nano 2015, 9, 5051–5062. [CrossRef] [PubMed]
Lee, C.-Y.; Hwang, J.-C.; Chueh, Y.-L.; Chang, T.-H.; Cheng, Y.-Y.; Lyu, P.-C. Hydrated bovine serum albumin
as the gate dielectric material for organic field-effect transistors. Org. Electron. 2013, 14, 2645–2651. [CrossRef]
Dumitru, L.; Manoli, K.; Magliulo, M.; Ligonzo, T.; Palazzo, G.; Torsi, L. A hydrogel capsule as gate dielectric
in flexible organic field-effect transistors. APL Mater. 2015, 3, 014904. [CrossRef]
Electronics 2016, 5, 9
24 of 24
108. Poghossian, A.; Bäcker, M.; Mayer, D.; Schöning, M.J. Gating capacitive field-effect sensors by the charge of
nanoparticle/molecule hybrids. Nanoscale 2015, 7, 1023–1031. [CrossRef] [PubMed]
109. Hammock, M.L.; Knopfmacher, O.; Naab, B.D.; Tok, J.B.H.; Bao, Z. Investigation of Protein Detection
Parameters Using Nanofunctionalized Organic Field-Effect Transistors. ACS Nano 2013, 7, 3970–3980.
[CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access
article distributed under the terms and conditions of the Creative Commons by Attribution
(CC-BY) license (http://creativecommons.org/licenses/by/4.0/).