Journal of Arid Environments 74 (2010) 928–932
Contents lists available at ScienceDirect
Journal of Arid Environments
journal homepage: www.elsevier.com/locate/jaridenv
Isotopic shift in an introduced population of gemsbok (Oryx gazella)
M.J. Marquez, W.J. Boecklen*
Department of Biology, Laboratory of Ecological Chemistry, MSC 3AF, New Mexico State University, Las Cruces, NM 88003-8001, USA
a r t i c l e i n f o
a b s t r a c t
Article history:
Received 13 September 2008
Received in revised form
5 January 2010
Accepted 15 January 2010
Available online 19 February 2010
We use isotope ratio mass spectrometry to examine the foraging ecology of an introduced population of
gemsbok, Oryx gazella, in New Mexico, USA. Gemsbok in New Mexico exhibit an isotopic shift in carbon
when compared to African gemsbok, suggesting that the introduced gemsbok include more shrubs
(C3 plants) in their diets than do native African gemsbok. New Mexican gemsbok did not differ significantly in isotopic signatures according to gender, maturity, or reproductive status; they did exhibit
significant patterns of fractionation in carbon and nitrogen isotopes between-tissue types (bone, muscle,
and hair). Stable isotope analysis indicated that C3 plants (shrubs and forbs) may comprise up to 44% of
the diets of gemsbok in New Mexico and that gemsbok diets may vary seasonally. These results are
consistent with previously published fecal analyses. Stable isotope analysis appears to be a useful tool for
rapid dietary analysis and may help wildlife managers to quickly evaluate the role of diet limitation or
expanded diet breadth on the population dynamics of introduced species, and to examine the role of
resource competition between native and introduced populations.
Ó 2010 Elsevier Ltd. All rights reserved.
Keywords:
Mammals
Stable isotopes
Foraging ecology
Introduced species
Chihuahuan desert
1. Introduction
There is much interest in the direct and indirect impacts of
introduced species on community composition and stability (Elton,
1958; Simberloff, 1991; Thompson, 1997), on ecosystem structure
and function (Ramakrishnan and Vitousek, 1989; Simberloff, 2005),
and on endangered native species (Fitzpatrick and Shaffer, 2007;
Mack et al., 2000; Pimentel et al., 2005). Much of this interest has
focused on introduced mammalian herbivores (Smit et al., 2001; de
Vos et al., 1956) and their potential to alter habitats and ecosystems
directly through browsing or trampling (Vasquez, 2002), or indirectly by changing plant species diversity (Wardle, 2001), patterns
of species interactions (Vasquez and Simberloff, 2003), and
nutrient dynamics (Wardle, 2001).
There are two major challenges facing invasion biologists: 1)
identifying characteristics of ecosystems that make them susceptible to invasion and characteristics of species that make them
successful invaders (Denslow, 2003; Jeschke and Strayer, 2005;
Kolar and Lodge, 2001); and 2) the development of predictive
models of invasion dynamics that lead to effective management
strategies (Davis et al., 2001; Simberloff et al., 2005). Both efforts
can be handicapped by a lack of detailed information on basic
natural history, such as habitat selection, reproductive biology,
physiological tolerances, and parasite and predation pressures
* Corresponding author. Tel.: þ1 575 646 5770.
E-mail address: wboeckle@nmsu.edu (W.J. Boecklen).
0140-1963/$ – see front matter Ó 2010 Elsevier Ltd. All rights reserved.
doi:10.1016/j.jaridenv.2010.01.004
(Simberloff, 2003; Simberloff et al., 2005). For introduced
mammalian herbivores, detailed information on foraging patterns
and diet width may be especially important in developing predictive models and effective management strategies.
The gemsbok (Oryx gazella) was introduced to the White Sands
Missile Range (hereafter, WSMR), New Mexico in 1969 by the New
Mexico Game and Fish Department as part of an exotic game animal
introduction program (Saiz and Decker, 1975). When first introduced into New Mexico, gemsbok were expected to utilize lower
elevation desert scrub and grassland areas within the designated
release sites in the Tularosa Basin (Wood et al., 1970). It is now
known that gemsbok occupy various terrain and habitat types
ranging from elevations of 1091 m to 2638 m (Burkett et al., 2002).
The population currently consists of 3000–5000 individuals (Rodden, pers. comm.) and occupies in excess of 15,000 km2 in southern
New Mexico (Bender et al., 2003). Possible mechanisms for the
rapid increase in the New Mexican gemsbok population include
predator and parasite release, competitive release, and an
expanded diet compared to native populations in southern Africa.
Stable isotope analysis has proven to be an effective technique in
diet analysis and in understanding trophic interactions within
communities (DeNiro and Epstein, 1978; Hobson et al., 1996; Yarnes
and Boecklen, 2006; Yarnes et al., 2005). For example, controlled
laboratory and field studies have demonstrated that carbon isotopic
signatures in herbivores reflect the proportions of C3 and C4 plants
in their diets (Cerling and Harris, 1999; DeNiro and Epstein, 1978;
Hobson et al., 1996). Here, we use stable isotope analysis of nitrogen
and carbon in hair, muscle, and bone collagen to examine the diet of
M.J. Marquez, W.J. Boecklen / Journal of Arid Environments 74 (2010) 928–932
gemsbok in New Mexico. We then compare the isotopic signatures
of New Mexican and African (published data) gemsbok and
demonstrate an isotopic shift for carbon in the introduced population. In addition, we test for differences in isotopic signatures
between genders, life stages, and reproductive states (females only)
within the New Mexican population. Lastly, we test for fraction
between-tissues types (see Martinez del Rio and Wolf, 2005;
Tieszen et al., 1983).
2. Materials and methods
2.1. Study area
The White Sands Missile Range encompasses approximately
8800 km2 and is bounded on the east by the foothills of the Sacramento Mountains and includes parts of the San Andres Mountains, Oscura Mountains, Tularosa Basin and Jornada del Muerto
Plain. This research was conducted in Rhodes Canyon in the
Tularosa Basin of the WSMR. Physical features include dry lakebeds,
canyons, grasslands, sand dunes, lava flows and mountain peaks.
The average annual precipitation is 31.3 cm, temperatures range
between 23 C and 44.4 C, and elevation ranges from 1189 to
2743 m. Vegetation on the WSMR is typical of southwestern semidesert shrub land and grassland. The northern Chihuahuan Desert
ecosystem is characterized by nearly equal annual aboveground net
primary productivity in grasslands and shrublands (Muldavin et al.,
2008). Aboveground net primary productivity in grasslands tends
to peak in summer and fall, while the shrublands tend to peak in
spring (Muldavin et al., 2008). Glasslands are composed almost
exclusively of C4 grasses (Cornelius et al., 1991; Muldavin et al.,
2008) including various threeawn (Aristida spp.), grama (Bouteloua
spp.), muhly (Muhlenbergia spp.), plains bristlegrass (Setaria
macrstachya), dropseeds (Sporobulus spp.), tridens and fluffgrass
(Tridens spp.), vine mesquite (Panicum spp.) and Hilaria spp. Forbs
are represented by C3 species, including sunflower (Conyza coulteri), pepperweed (Lepidium spp.), globemallow (Sphaeralcea spp.),
and buffalogourd (Cucurbita foetidissima); and by C4 species,
including russianthistle (Salsola kali) and seepweed (Suaeda spp.).
Shrublands are dominated by C3 species, including creosotebush
(Larrea tridentata), mesquite (Prosopis spp.), yucca (Yucca spp.), and
tamarisk (Tamarix gallica). Shrublands also contain C4 species,
including saltbush (Atriplex spp.), and CAM species, including
pricklypear (Opuntia engelmannii) and cholla (Cylindropuntia spp.).
A list of plants on the WSMR can be found in Saiz and Decker
(1975), Fletcher (2000) and Dick-Peddie (1993).
2.2. Sample collection and tissue preparation
A total of 71 animals were sampled throughout the 2006–2007
hunting season (September 16 to March 4). A total of 65 hair
samples, 59 bone samples, and 31 muscle samples were acquired.
Whenever possible, gender, age class, and reproductive status were
documented.
Lipid extractions were performed on all samples of hair, muscle,
and bone using a modified version of the procedure outlined by
Blight and Dyer (1959). Hair preparation was as follows: several
strands of hair from each animal were cleaned by ultra-sonification
twice in a 1:2 v/v methanol: chloroform solution for 20 min to
remove surface contaminants and sebum lipids. The strands were
then rinsed with deionized-distilled water twice for 20 min after
each cleaning. The hairs were then allowed to air dry in a fume
hood for 48 h. Next, the samples were ground with a mortar and
pestle using liquid N and stored in micro-centrifuge tubes. Each
sample was weighed on an electronic microbalance to approximately 0.5 mg.
929
Muscle was prepared for lipid extraction by first freeze-drying
samples in plastic tubes for 48 h. One ml of chloroform and 1 ml of
methanol were then added, vortexed for 30 s after which, another 1
ml of chloroform was added. The sample was vortexed again for 10
s and then sonicated for 20 min. One ml of deionized water was
then added, vortexed for an additional 10 s and then sonicated for
20 s. This procedure was performed twice to assure the removal of
all lipids. The samples were dried under a fume hood for 48 h. The
lipid free muscle samples were ground to a fine powder using
a mortar and pestle, and approximately 0.5 mg of each sample were
placed into foil capsules and stored in a desiccator.
Powdered bone samples were acquired by drilling a portion of
the leg bone. The area chosen for sampling was cleaned by first
removing all skin and muscle attached to the bone and then ‘‘polishing’’ the area with a diamond-abrasive spear-shaped rotary tool
(Gilles St-Jean, pers. comm.). Several layers of bone were removed
using a 1.2 mm diamond-tipped rotary tool; approximately 2–3 g of
powdered bone was collected. To begin the analysis of bone
collagen, it is imperative to remove all traces of lipids in the bone
since lipids will interfere with the C/N ratio of the bone collagen
(Copley et al., 2004; O’Connel and Hedges, 2001; Post et al., 2007).
To begin lipid extraction, approximately 2 g of the bone sample was
placed into plastic tubes. The procedure follows the same method
as for the muscle samples except that 2 ml of chloroform, methanol
and water were used instead of 1 ml and the procedure was only
performed once.
Collagen extraction followed the procedure outlined by Copley
et al. (2004). Approximately 0.2 g of powdered bone samples was
demineralized in plastic tubes, with 10 ml of 0.5 M HCl for 24 h at
room temperature. The samples were agitated three times during
this period. They were then rinsed twice with 10 ml of deionized
water and centrifuged between washes to minimize sample loss.
Next, the residues were gelatinized in 10 ml of 0.001 M HCl at 75 C
for 48 h and then freeze dried. After the completion of both lipid
and collagen extractions approximately 0.5 mg of sample was
loaded into foil capsules and stored in a desiccator.
2.3. Stable isotope analysis
All isotopic analyses of samples were performed in the Laboratory for Ecological Chemistry (LEC) at New Mexico State University.
Samples were combusted using a Costech Analytical Elemental
Analyzer (Valencia, CA) and introduced to a ThermoFinnigan DeltaPlus XP Isotopic Ratio Mass Spectrometer (Waltham, MA) in
continuous-flow through a ConFlo III Interface. Results are
described in delta (d) notation in measures of ‘‘per mil’’ (&) or per
parts per thousand. Results are reported as the difference between
the isotopic ratio of the sample and appropriate international
standards: ‘‘VPDB’’ (Vienna Pee Dee Belemnite, a carbonate rock)
for 13C and ‘‘Air’’ (atmospheric nitrogen) for 15N. The results are
then standardized using the following formula:
d13 C or d15 N ¼
.
i
h
Rstandard 1000;
Rsample Rstandard
where R ¼ 13C/12C or 15N/14N. All analyses are traceable to the NIST
(National Institute of Standards and Technology, Washington, DC)
isotopic standard reference materials IAEA-N1, IAEA-N2, USGS24,
USGS32 and NBS-19.
2.4. Statistical analyses
Isotopic differences in carbon and nitrogen between African and
New Mexican gemsbok were analyzed using one-sample t-tests,
where the null hypotheses were constructed using published mean
delta values for the African population: 9.5& for bone d13C
930
M.J. Marquez, W.J. Boecklen / Journal of Arid Environments 74 (2010) 928–932
(Ambrose and DeNiro, 1986), 10.3& for hair d13C (Cerling et al.,
2003), and 8.9& for bone d15N (Ambrose and DeNiro, 1986). African
gemsbok were sampled from the Tsavo and Turkana regions of East
Africa (Cerling et al., 2003), and from the arid shore of Lake Turkana
(Ambrose and DeNiro, 1986). Variation in d13C and d15N values in
the New Mexican population with respect to gender, age class, and
reproductive status were analyzed using one-way analyses of
variance. Isotopic differences for C and N between different tissues
(hair, muscle and bone) were analyzed using paired t-tests. Analyses were conducted using Systat Version 11 (SSI, Richmond, CA).
3. Results
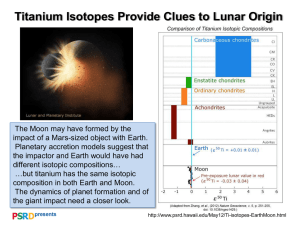
Fig. 1. d13C values associated with C4 and C3 plants and carbon isotopic signatures of
New Mexican (NM) and African (SA) gemsbok.
A total of 71 gemsbok were sampled: 32 males (25 adults and 7
sub-adults) and 39 females (33 adults and 6 sub-adults). Out of the
33 adult females, 14 were pregnant. Adult males were larger, on
average, than were adult females (247.9 cm and 206.1 cm mean
shoulder height, respectively); however, this difference was not
significant as determined by a one-way analysis of variance (p ¼
0.357). New Mexican gemsbok did not differ significantly in
isotopic signatures (d13C or d15N) according to gender, maturity, or
reproductive status (Table 1). Consequently, we pooled all New
Mexican gemsbok for the analyses that follow.
New Mexican gemsbok exhibited significant patterns of fractionation in carbon and nitrogen isotopes (differences in isotopic
values) between-tissue types. For carbon, d13C values differed
significantly (p < 0.001), as determined by paired t-tests, between
bone and muscle, between bone and hair, and between muscle and
hair. For nitrogen, d15N values differed significantly between bone
and hair (p < 0.001) and between muscle and hair (p < 0.001);
differences between bone and muscle were only marginally
significant (p ¼ 0.074).
New Mexican gemsbok differed significantly from African
gemsbok in isotopic signatures for carbon, and while still within the
range characteristic of C4 plants, they were shifted, relative to the
African values, toward the C3 end of the spectrum (Fig. 1). For
example, New Mexican gemsbok exhibited averages of 13.8& for
d13C in bone and 17.5& for d13C in hair, while African gemsbok
averaged 9.5& (Ambrose and DeNiro, 1986) and 10.3& (Cerling
et al., 2003), respectively. These differences were highly significant
(p < 0.001) as determined by the one-sample t-tests using the
African values as null hypotheses. In contrast, mean values of d15N
in bone for New Mexican gemsbok (8.7&) and African gemsbok
(8.9&; Ambrose and DeNiro, 1986) did not differ significantly (p ¼
0.218).
4. Discussion
Stable isotope analysis of herbivore tissue has been used to
estimate the relative proportions of C3 and C4 plants in diets of
numerous species (Ambrose and DeNiro, 1986; Cerling et al., 2003).
The method appears to be quite reliable, as diet reconstructions
based on differences in carbon isotope signatures between C3 and
C4 photosynthesis has been verified experimentally (Cerling and
Harris, 1999; DeNiro and Epstein, 1978; Hobson et al., 1996). In fact,
animal tissue samples have been shown to differ from the isotopic
composition of diet by as little as 1& (Hobson and Clark, 1992;
Tieszen et al., 1983). In other words, the isotopic signatures of
consumers typically exhibit d13C values close to the d13C values of
the actual diet, providing an accurate measure of diet composition.
Diet analyses of native African gemsbok indicate that gemsbok
are primarily grazers that will occasionally consume forbs and
browse. Gemsbok have been classified as grazers (Zhaowen and
Takatsuki, 1999), as variable grazers (Gagnon and Chew, 2000), as
grass-mixed grass feeders (Spencer, 1995), or as hypergrazers
(greater than 95% C4 grasses) (Cerling et al., 2003). Direct observational estimates of the grazing component of gemsbok diets in
the Kalahari are as much as 93.6% (Knight, 1991).
Isotopic analyses of native gemsbok in Africa strongly indicate
a diet consisting largely of C4 grasses (Ambrose and DeNiro, 1986;
Cerling et al., 2003). Our analysis of an introduced gemsbok population in New Mexico indicates a significant shift in carbon isotope
signatures and suggests that C3 plants (shrubs) comprise a significant portion of the diets of these animals. These results are
consistent with the observations of Reid and Patrick (1983) who
observed gemsbok on the White Sands National Monument feeding
on C3 forbes, including globemallow (Sphaeralcea spp.), sunflower
(C. coulteri), and buffalogourd (C. foetidissima). Of course, an alternative explanation is that the isotopic signatures of New Mexican
C4 plants are greatly different from those in southern Africa.
However, we feel that is unlikely as 11 of the 33 plant genera eaten
by gemsbok in South Africa are also found in New Mexico (White,
1967).
We can estimate the size of the C3 component in the diets of
New Mexican gemsbok as follows:
13
13
13
NM d C ¼ pC3 d C þ ð1 pÞSA d C ;
where p is the fraction of C3 plants in the diet; C3(d13C) is the
isotopic signature of C3 plants; and NM(d13C) and SA(d13C) are the
observed isotopic signatures for New Mexican and southern African
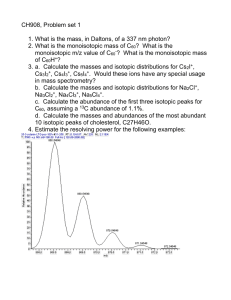
Table 1
Isotopic values (mean standard error) by tissue type for male and female (pregnant and non-pregnant) Oryx. The category Overall represents males and females combined.
The first column of p-values are the results of one-way analyses of variance testing for differences between gender, the second column of p-values represents tests for
differences between pregnant and non-pregnant females.
Overall
Bone d13C
Bone d15N
Muscle d13C
Muscle d15N
Hair d13C
Hair d15N
13.789
8.681
18.587
8.288
17.528
9.684
Male
0.164
0.176
0.243
0.212
0.222
0.184
13.832
8.765
18.507
8.234
17.521
9.801
Female
0.257
0.261
0.339
0.319
0.307
0.293
13.757
8.619
18.610
8.274
17.533
9.591
0.217
0.240
0.325
0.262
0.320
0.237
p-value
Pregnant
0.826
0.686
0.764
0.817
0.979
0.575
14.056
8.833
18.061
8.459
18.243
9.893
Non-pregnant
0.437
0.345
0.663
0.655
0.487
0.361
13.569
8.485
18.928
8.278
17.114
9.412
0.221
0.328
0.414
0.326
0.401
0.310
p-value
0.282
0.489
0.272
0.785
0.088
0.334
M.J. Marquez, W.J. Boecklen / Journal of Arid Environments 74 (2010) 928–932
gemsbok, respectively. The model basically asks, ‘‘If we start with
an African gemsbok, what percentage of the diet must be C3 plants
to achieve an isotopic signature characteristic of New Mexican
gemsbok?’’ If we assume a mode of C3(d13C) ¼ 26.7& (see Vogel,
1980), then the model gives p ¼ 0.25 for bone and p ¼ 0.44 for hair.
In other words, C3 plants represent between 25% and 44% of the
diet of New Mexican gemsbok.
The difference in the above estimates (25% based on bone and
44% based on hair) suggests that there may be a seasonal component to the composition of gemsbok diets. It is well established that
different tissues, even within the same organisms, may exhibit
distinct isotopic signatures (Martinez del Rio and Wolf, 2005;
Tieszen et al., 1983). These between-tissue differences, or fractionation, are due, in part, to different rates of turnover within
tissue types. It is often assumed that tissues with distinct rates of
turnover represent diets integrated over various temporal scales
(Dalerum and Angerbjorn, 2005; Tieszen et al., 1983; Wolf et al.,
2009). For example, isotopic signatures in bone may integrate over
the course of a year, hair over a few months, and muscle over
several weeks. If we adopt this assumption regarding tissue fractionation, then our results suggest that New Mexican gemsbok
incorporate C3 plants throughout the year and that C3 plants
become more prevalent in the diet during winter and early spring
(see Fig. 1). This pattern corresponds to increased aboveground net
primary productivity of Chihuahuan Desert shrublands relative to
grasslands in colder seasons (Muldavin et al., 2008).
An alternate explanation for tissue fractionation is isotopic
routing (Jim et al., 2006). For example, the observed differences in
d13C values between bone and hair may be due to differential
incorporation of amino acids that vary in isotopic composition. Jim
et al. (2006) report that individual amino acids in diets and in bone
collagen can vary by as much as 16 parts per mil, and provide
experimental evidence of routing of particular amino acids to bone
collagen. However, Jim et al. (2006) also demonstrate that the
isotopic composition of particular amino acids in bone collagen and
differences in d13C values between diet and amino acids does vary
with diet in both magnitude and direction. For example, glycine,
which constitutes approximately 33% of bone collagen, varies from
15.9 to 6.6 d13C across diets, and is depleted in 15C relative to diet
in some diets, but is enriched in others. Given the dynamic and
complex nature of isotopic routing, particularity with respect to
variation in diet, it is difficult to assess the degree to which isotopic
routing may confound our interpretation of seasonal differences in
gemsbok diets. Of course, these competing explanations of tissue
fractionation (and the assumption of different temporal integration
by tissue type) are best addressed by controlled laboratory experiments, which are beyond the scope of the present study.
Our major conclusion, that New Mexican gemsbok incorporate
a significant fraction of C3 plants (shrubs) into their diets and that
diet composition may vary seasonally, is supported by fecal pellet
analyses. For example, Saiz and Decker (1975) conducted fecal
analyses and concluded that C3 shrubs make up 47.1% of the diet of
the gemsbok on the WSMR, while Fletcher (2000) reports 38%
shrubs. In addition, Dye (1998) concluded that gemsbok are
primarily grazers with diets consisting of 61.3% grasses and 22.9%
shrubs. Reid and Patrick (1983) provided evidence from observation and scat analysis that gemsbok diets consisted of 80% grasses
on the WSNM. Moreover, Smith et al. (1998), Fletcher (2000) and
Dye (1998), examined seasonal variation in gemsbok diets using
classifications of warm/wet, cool/dry and warm/dry seasons. Smith
et al. (1998) and Dye (1998) observed that the relative abundance of
shrubs increased in the diet in the cool/dry months, while Fletcher
(2000) found little seasonal variation.
We found no significant differences among gemsbok in isotopic
signatures between genders, between age classes, or between
931
reproductive classes. These results provide little evidence of
intraspecific resource partitioning among gemsbok in New Mexico.
Ruckstuhl and Neuhaus (2009) examined foraging behavior and
activity budgets of mixed-sex herds of gemsbok in Etosha National
Park, Namibia. They observed no significant effects of gender or
reproductive status on feeding behavior (bite rates, step rates, and
changes in direction).
4.1. Management implications
The New Mexican gemsbok population has increased rapidly
since their introduction in 1969. An initial introduction of 93
animals has resulted in a current population of 3000–6000 (Bender
et al., 2003). Effective management of this population requires an
understanding of the potential mechanisms underlying population
dynamics. Potential mechanisms for the rapid increase in the New
Mexican gemsbok population include predator and parasite
release, competitive release, and an expansion in diet breadth.
Stable isotope analysis indicates a potential role for diet breadth
expansion as New Mexican gemsbok incorporate significantly more
shrubs and forbs in their diets than do their African counterparts. Of
course, this hypothesis will require further investigation. Gasaway
et al. (1996) investigated resource limitation as a potential mechanism for persistently low populations of gemsbok in Etosha
National Park, Namibia. They found little evidence that gemsbok
populations were limited by resource availability; they suggested
a primary role of high adult and yearling mortality resulting from
anthrax and predation.
The rapid increase in the New Mexican gemsbok population
coincides with population declines of native ungulates, including
desert mule deer (Odocoileus hemionus crooki) and desert bighorn
sheep (Ovis Canadensis mexicana) (Bender et al., 2003). Important
questions facing wildlife biologists when introduced species
interact with native species is determining to what degree the
population dynamics of species are related and what are the
mechanisms underlying these interactions.
Introduced gemsbok could impact native ungulates directly
through resource competition or indirectly through habitat alteration (Burkett et al., 2002) or through transmission of parasites
(Bender et al., 2003). Stable isotope analysis of introduced gemsbok
suggests a potential role for resource competition in that C3 plant
(shrubs and forbs) may constitute as much as 44% of the diet.
Rumen analyses indicates that mule deer diets are dominated by
browse species (75% shrubs, 17% forbs and 2.2% grasses) (Boeker
et al., 1972), while fecal microhistology indicates 65% shrubs and
15% grasses (Dye, 1998). Fecal analyses and direct observations
indicate that browse is the most important dietary component for
the desert bighorn sheep (64%) followed by forbs (23%) and then
grasses (13%) (Miller and Gaud, 1989). Whether there is, in fact,
sufficient dietary overlap between gemsbok and mule deer and
desert bighorn sheep to result in negative population dynamics
owing to resource competition must await further study.
5. Conclusion
Stable isotope analysis of hair, muscle, and bone tissue collected
from an introduced population of gemsbok indicated that New
Mexico gemsbok are primarily grazers but that they incorporated
significantly more C3 plants (shrubs and forbs) in their diets than do
African gemsbok. The percentage of C3 plants in the diet vary
seasonally and corresponded to seasonal patterns of net primary
production in the Chihuahuan Desert ecosystem.
Stable isotope analysis appears to be a useful tool for wildlife
biologists, especially for those managing introduced species. It
allows for rapid characterization of diets in native and introduced
932
M.J. Marquez, W.J. Boecklen / Journal of Arid Environments 74 (2010) 928–932
populations. Such information is critical in assessing the role of diet
limitation or expanded diet breadth on the dynamics of introduced
populations. In addition, stable isotope analysis can be used to
investigate potential impacts to native species through resource
competition.
Acknowledgments
This work was completed in partial fulfillment of the requirements of a Masters Degree at New Mexico State University by M.
Marquez. The work was supported, in part, by NSF grants DEB
0129630 and DMS 0337789 to W. Boecklen.
References
Ambrose, S.H., DeNiro, M.J., 1986. The isotopic ecology of East African mammals.
Oecologia 69, 395–406.
Bender, L.C., Li, H., Thompson, B.C., Morrow, P.C., Valdez, R., 2003. Infectious disease
survey of gemsbok in New Mexico. Journal of Wildlife Diseases 39 (4), 772–778.
Boeker, E.L., Scott, V.E., Reynolds, H.G., Donaldson, B.A., 1972. Seasonal food habits of
mule deer in southwestern New Mexico. Journal of Wildlife Management 36,
56–63.
Burkett, D.W., Valdez, R., Thompson, B.C., Boykin, K.G., 2002. Gemsbok: the
management challenge of an exotic ungulate in the American Southwest. In:
Ebedes, H., Reilly, B., van Hoven, W., Penzhorn, B. (Eds.), Sustainable UtilizationdConservation in Practice. Proceedings of the Fifth Wildlife Ranching
Symposium. South African Game Rancher’s Organization, Pretoria, South Africa,
pp. 166–171.
Blight, E.G., Dyer, W.J., 1959. A rapid method of total lipid extraction and purification. Canadian Journal of Biochemistry and Physiology 37, 911–917.
Cerling, T.E., Harris, J.M., 1999. Carbon isotope fractionation between diet and
bioapatite in ungulate mammals and implications for ecological paleoecological
studies. Oecologia 120, 347–363.
Cerling, T.E., Harris, J.M., Passey, B.H., 2003. Diets of East African bovidae based on
stable isotope analysis. Journal of Mammology 84 (2), 456–470.
Copley, M.S., Jim, S., Jones, V., Rose, P., Clapham, A., Edwards, D.N., Horton, M.,
Rowley-Conwy, P., Evershed, R.P., 2004. Short- and long-term foraging and
foddering strategies of domesticated animals from Qasr Ibrim, Egypt. Journal of
Archaeological Science 31, 1273–1286.
Cornelius, J.M., Kemp, P.R., Ludwig, J.A., Cunningham, G.L., 1991. The distribution of
vascular plant species and guilds in space and time along a desert gradient.
Journal of Vegetation Science 2, 59–72.
Dalerum, F., Angerbjorn, A., 2005. Resolving temporal variation in vertebrate diets
using naturally occurring stable isotopes. Oecologia 144, 647–658.
Davis, M.A., Thompson, K., Grime, J.P., 2001. Charles S. Elton and the dissociation of
invasion ecology from the rest of ecology. Diversity and Distributions 7, 97–102.
Denslow, J.S., 2003. Weeds in paradise: thoughts on the invisibility of tropical
islands. Missouri Botanical Garden 90, 119–127.
DeNiro, M.J., Epstein, S., 1978. Influence of diet on the distribution of carbon
isotopes in animals. Geochimica et Cosmochimica Acta 42, 495–506.
Dick-Peddie, W.A., 1993. New Mexico Vegetation, Past, Present, and Future.
University of New Mexico Press, Albuquerque, NM.
Dye, J., 1998. Gemsbok and Mule Deer Diets in Southern New Mexico. M.S. thesis,
New Mexico State University, Las Cruces, New Mexico.
Elton, C.S., 1958. The Ecology of Invasions by Animals and Plants. Methuen, London.
Fitzpatrick, B.M., Shaffer, H.B., 2007. Hybrid vigor between native and introduced
salamanders raises new challenges for conservation. Proceedings of the
National Academy of Sciences 104 (40), 15793–15798.
Fletcher, T.L., 2000. Diets of Gemsbok in Creosote Shrubland and Great Basin Conifer
Woodland in South Central New Mexico. Masters thesis, New Mexico State
University, Las Cruces, New Mexico.
Gagnon, M., Chew, A.E., 2000. Dietary preferences in extant African Bovidae. Journal
of Mammalogy 81, 490–511.
Gasaway, W.C., Gasaway, K.T., Berry, H.H., 1996. Persistent low densities of plains
ungulates in Etosha National Park, Namibia: testing the food-regulating
hypothesis. Canadian Journal of Zoology 74, 1556–1572.
Hobson, K.A., Clark, R.G., 1992. Assessing avian diets using stable isotopes II: factors
influencing diet-tissue fractionation. The Condor 94 (1), 189–197.
Hobson, K.A., Schell, D.M., Renouf, D., Noseworthy, E., 1996. Stable carbon and
nitrogen isotopic fractionation between diet and tissue of captive seals:
implications for dietary reconstructions involving marine mammals. Canadian
Journal of Fisheries and Aquatic Sciences 53, 528–533.
Jeschke, J.M., Strayer, D.L., 2005. Invasion success of vertebrates in Europe and North
America. Proceedings of the National Academy of Sciences 102 (20), 7198–7202.
Jim, S., Jones, V., Ambrose, S.H., Evershed, R.P., 2006. Quantifying dietary macronutrient sources of carbon for bone collagen biosynthesis using natural abundance stable carbon isotope analysis. British Journal of Nutrition 95, 1055–1062.
Kolar, C.S., Lodge, D.M., 2001. Progress in invasion biology: predicting invaders.
Trends in Ecology and Evolution 16 (4), 199–204.
Knight, M.H., 1991. Ecology of the Gemsbok Oryx gazella gazella (Linnaeus) and Blue
Wildebeest Connochaetes taurinus (Burchell) in the Southern Kalahari. Dissertation, University of Pretoria, Pretoria, South Africa.
Mack, R.N., Simberloff, D., Lonsdale, W.M., Evans, H., Clout, M., Bazzaz, F.A., 2000.
Biotic invasions: causes, epidemiology, global consequences, and control.
Ecological Applications 10 (3), 689–710.
Martinez del Rio, C., Wolf, B.O., 2005. Mass-balance models for isotopic ecology. In:
Starck, J.M., Wang, T. (Eds.), Physiological and Ecological Adaptations to Feeding
in Vertebrates. Science Publishers, Enfield, New Hampshire.
Miller, G.D., Gaud, W.S., 1989. Composition and variability of desert bighorn sheep
diets. Journal of Wildlife Management 53, 597–606.
Muldavin, E.H., Moore, D.I., Collins, S.L., Wetherill, K.R., Lightfoot, D.C., 2008.
Aboveground net primary production dynamics in a northern Chihuahuan
Desert ecosystem. Oecologia 155, 123–132.
O’Connel, T.C., Hedges, R.E.M., 2001. Isotopic comparison of hair, nail and bone:
modern analysis. Journal of Archaeological Science 28, 1247–1255.
Pimentel, D., Zuniga, R., Morrison, D., 2005. Update on the environmental and
economic costs associated with alien-invasive species in the United States.
Ecological Economics 52 (3), 273–288.
Post, D.M., Layman, C.A., Arrington, D.A., Takimoto, G., Quattrochi, J., Montana, C.G.,
2007. Getting to the fat of the matter: models, methods and assumptions for
dealing with lipids in stable isotope analysis. Oecologia 152, 179–189.
Ramakrishnan, P.S., Vitousek, P.M., 1989. Ecosystem-level processes and the
consequences of biological invasions. In: Drake, J.A., Mooney, H.A., di Castri, F.,
Groves, R.H., Kruger, F.J., Rejmanek, M., Williamson, M. (Eds.), Biological Invasions: a Global Perspective. John Wiley and Sons, New York, NY, pp. 281–296.
Reid, W.H., Patrick, G.R., 1983. Gemsbok (Oryx gazella) in White Sands national
monument. The Southwestern Naturalist 28 (1), 97–99.
Rucksthul, K.E., Neuhaus, P., 2009. Activity budgets and sociality in a monomorphic
ungulate: the African oryx (Oryx gazella). Canadian Journal of Zoology 87, 165–174.
Saiz, R.B., Decker, E., 1975. Ecology and Behavior of the Gemsbok on the White
Sands Missile Range, New Mexico. Prepared for the New Mexico Department of
Game and Fish, Federal Aid Project W-111-R-8.
Simberloff, D., 1991. Keystone species and community effects of biological introductions. In: Ginsburg, L.R. (Ed.), Assessing Ecological Risks of Biotechnology.
Butterworth-Heinemann, Boston, MA, pp. 1–19.
Simberloff, D., 2003. How much information on population biology is needed to
manage Introduced species? Conservation Biology 17 (1), 83–92.
Simberloff, D., 2005. The politics of assessing risk for biological invasions: the USA
as a case study. Trends in Ecology and Evolution 20 (5), 216–222.
Simberloff, D., Parker, I.M., Windle, P.N., 2005. Introduced species policy, management, and future research needs. Frontiers in Ecology and the Environment 3
(1), 12–20.
Smit, R., Bokdam, J., den Ouden, J., Olff, H., Schot-Opschoor, H., Schrijvers, M., 2001.
Effects of introduction and exclusion of large herbivores on small rodent
communities. Plant Ecology 155, 119–127.
Smith, C., Valdez, R., Holechek, J.L., Zwank, P.J., Cardenas, M., 1998. Diets of native
and non-native ungulates in Southcentral New Mexico. The Southwestern
Naturalist 43 (2), 163–169.
Spencer, L.M., 1995. Morphological correlates of dietary resource partitioning in the
African Bovidae. Journal of Mammalogy 76, 448–471.
Thompson, J.N., 1997. Conserving interaction biodiversity. In: Pickett, S.T.A.,
Ostfeld, R.S., Shachak, M., Likens, G.E. (Eds.), The Ecological Basis of Conservation: Heterogeneity, Ecosystems, and Biodiversity. Chapman and Hall, New
York, NY, pp. 285–293.
Tieszen, L.L., Boutton, T.W., Tesdahl, K.G., Slade, N.A., 1983. Fractionation and
turnover of stable carbon isotopes in animal tissues: implications for d13C
analysis of diet. Oecologia 57, 32–37.
Vasquez, D.P., 2002. Multiple effects of introduced mammalian herbivores in
a temperate forest. Biological Invasions 4, 175–191.
Vasquez, D.P., Simberloff, D., 2003. Changes in interaction biodiversity induced by
an introduced ungulate. Ecology Letters 6, 1077–1083.
Vogel, J.C., 1980. Fractionation of Carbon Isotopes During Photosynthesis. SpringerVerlag, Heidelberg.
de Vos, A., Manville, R.H., van Gelder, R.G., 1956. Introduced mammals and their
influence on native biota. Zoologica (New York Zoological Society) 41, 163–194.
Wardle, D.A., 2001. Introduced browsing mammals in New Zealand natural forests:
aboveground and belowground consequences. Ecological Monographs 71, 587–614.
White, R.J., 1967. Preliminary Investigation of the Factors Involved in Stocking
Greater Kudu and Gemsbok in New Mexico. M.S. thesis, New Mexico State
University, Las Cruces, New Mexico.
Wolf, N., Carleton, S.A., Carlos Martı́nez del Rio, C., 2009. Ten years of experimental
animal isotopic ecology. Functional Ecology 23, 17–26.
Wood, J.E., White, R.J., Durham, J.L., 1970. Investigations Preliminary to the Release
of Exotic Ungulates in New Mexico. In: Bulletin No. 13. New Mexico Department
of Game and Fish, Santa Fe, New Mexico, pp. 1–58.
Yarnes, C.T., Rockwell, J., Boecklen, W.J., 2005. Patterns of the trophic shift in d15N
and d13C of a cynipid gall wasp community (Antron acraspiformis) in Quercus
turbinella (Greene). Environmental Entomology 34, 1471–1476.
Yarnes, C., Boecklen, W.J., 2006. Trophic shift in d15N and d13C through galling
arthropod communities: estimates from Quercus turbinella and Salix exigua. In:
Ozaki, K., Yukawa, J., Ohgushi, T., Price, P.W. (Eds.), Galling Arthropods and Their
Associates. Springer-Verlag, Tokyo, pp. 43–53.
Zhaowen, J., Takatsuki, S., 1999. Constraints on feeding type in ruminants: a case for
morphology over phylogeny. Mammal Study 24, 79–89.