CH908, Problem set 1
advertisement
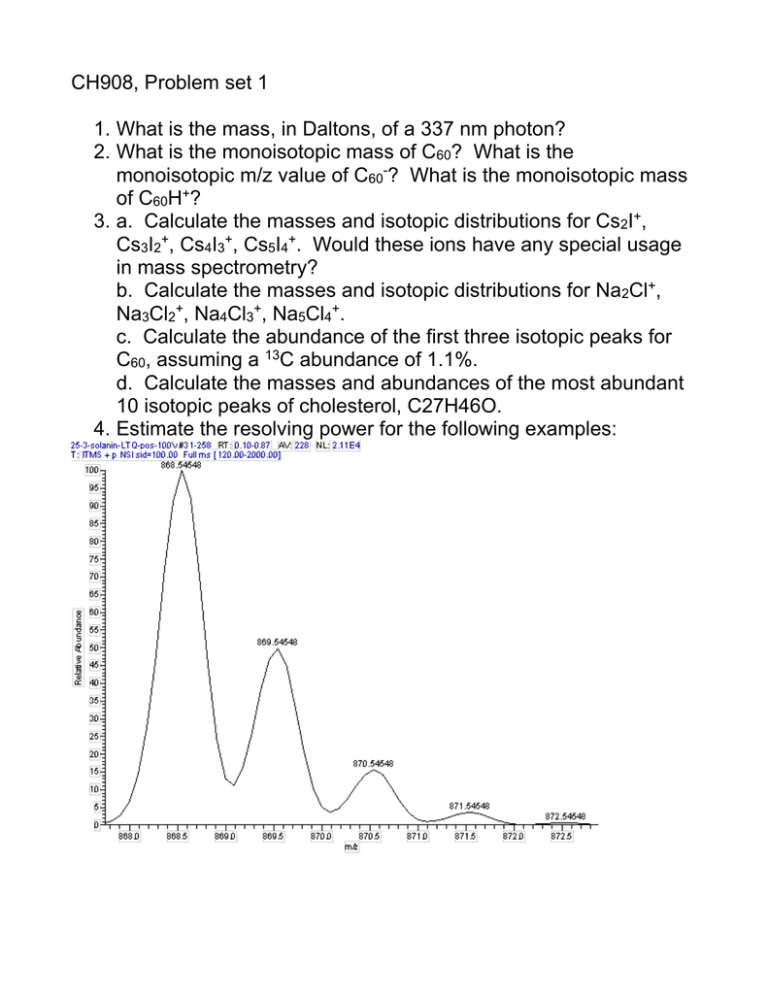
CH908, Problem set 1 1. What is the mass, in Daltons, of a 337 nm photon? 2. What is the monoisotopic mass of C60? What is the monoisotopic m/z value of C60-? What is the monoisotopic mass of C60H+? 3. a. Calculate the masses and isotopic distributions for Cs2I+, Cs3I2+, Cs4I3+, Cs5I4+. Would these ions have any special usage in mass spectrometry? b. Calculate the masses and isotopic distributions for Na2Cl+, Na3Cl2+, Na4Cl3+, Na5Cl4+. c. Calculate the abundance of the first three isotopic peaks for C60, assuming a 13C abundance of 1.1%. d. Calculate the masses and abundances of the most abundant 10 isotopic peaks of cholesterol, C27H46O. 4. Estimate the resolving power for the following examples: 5. What elemental compositions are possible for a positive ion molecule of mass 386.3542 +/- 5 ppm? What about a neutral fragment loss of 43.0184 +/- 100 ppm? Assume Carbon, Hydrogen, Oxygen, Nitrogen, and Sulfur. Explain how you determine the possible compositions. 6. Calculate the R+DB of each elemental composition listed in question 5. 7. How many hydrogen, oxygen, carbon, nitrogen, chlorine, bromine, phosphorus, and sulfur atoms are possible in the following example isotopic patterns?