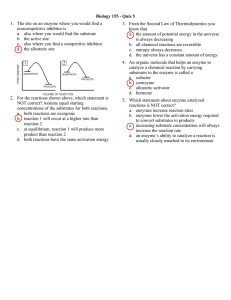
Problem Set 6 1. The following table indicates the rate at which a
advertisement

Problem Set 6 1. The following table indicates the rate at which a substrate reacts as catalyzed by an enzyme that follows Michaelis-Menten mechanism: 1) in the absence of inhibitor; 2) and 3) in the presence of 10 mM concentration, respectively, of each of the two inhibitors. Assume [E]T is the same for all reactions. [S] (mM) (2) vo (μM/s) (3) vo (μM/s) (1) vo (μM/s) 1 2.5 1.17 0.77 2 4.0 2.10 1.25 5 6.3 4.00 2.00 10 7.6 5.7 2.5 20 9.0 7.2 2.86 (a) Determine the Km and Vmax for the enzyme. For each inhibitor determine the type of inhibition, and the enzyme’s Km and Vmax values with each inhibitor. (b) For [S] = 5 mM, what fraction of the enzyme molecules have a bound substrate in the absence of inhibitor? 2. The Km of a Michaelis-Menten enzyme for a substrate is 1.0x10-4 M. At a substrate concentration of 0.20 M, vo=43 μM/min for a certain enzyme concentration. What is the value of vo for this enzyme at a substrate concentration of 0.02 M? 3. For the following reactions tell what major class of enzymes could be involved in catalyzing the reaction of substrate to product. What cofactors could be involved in reactions (a) and (b)? (a) L-lactate → pyruvate (b) α-D-glucose → glucose-6-phosphate (c) L-alanine → D-alanine 4. A ligand acts on an allosteric enzyme by decreasing the Km value of the enzyme. What class of allosteric enzyme is this, and is the ligand an activator or an inhibitor of the enzyme? 5. Briefly explain the specificity of an enzyme for a given substrate and also explain the specificity of enzyme reactions. 6. The covalent catalytic mechanism of an enzyme depends on a single active-site Cys whose pK is 8.0. A mutation in a nearby residue alters the microenvironment so that this pK increases to 10. Would the mutation cause the reaction rate to increase or decrease. Explain. 7. In a bisubstrate reaction, a small amount of the first product P1 is isotopically labeled and added to the enzyme and the first substrate A. No B or P2 is present. Will A become isotopically labeled if the reaction follows a Ping Pong mechanism or a Sequential mechanism? 1. a) Enzyme: Vmax= 10 Km = 3.0 Inhibitor 1: Vmax= 10 Km = 7.54 Inhibitor 2: Vmax = 3.33 Km = 3.33 competitive uncompetitive 1.4 y = 0.9989x + 0.3001 1.2 enzyme 1 inhibitor 1 y = 0.7541x + 0.1 0.8 inhibitor 2 Linear (inhibitor 2) 0.6 0.4 Linear (inhibitor 1) y = 0.3017x + 0.0987 Linear (enzyme) 0.2 0 0 0.5 1 1.5 b) [ES]/[E]T can be estimated by vo/vmax which is equal to 62.5% 2. [S] Km+[S]