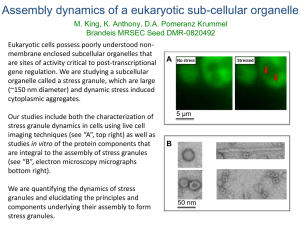
From www.bloodjournal.org by guest on September 30, 2016. For personal use only.
PHAGOCYTES
Haptoglobin is synthesized during granulocyte differentiation, stored in specific
granules, and released by neutrophils in response to activation
Kim Theilgaard-Mönch, Lars C. Jacobsen, Marianne J. Nielsen, Thomas Rasmussen, Lene Udby, Maged Gharib,
Peter D. Arkwright, Adrian F. Gombart, Jero Calafat, Søren K. Moestrup, Bo T. Porse, and Niels Borregaard
Haptoglobin (Hp) is a plasma protein synthesized primarily by hepatocytes. It exerts a broad range of anti-inflammatory
activities and acts indirectly as a bacteriostatic agent and an antioxidant by virtue
of its ability to bind free hemoglobin (Hb)
and to facilitate its immediate clearance
by macrophages. We identified Hp as a
novel specific granule protein of neutrophils by means of immunoelectron microscopy, subcellular fractionation, and
exocytosis studies. Consistent with these
findings, blood cells from a patient with
specific granule deficiency (SGD) lacked
neutrophil-derived Hp. Neutrophils contained a large amount of highly glycosylated Hp (-chain 45-65 kDa) synthesized
in neutrophil precursors and stored in
specific granules and a small amount of
Hp (-chain 39 kDa) endocytosed from
plasma and stored in secretory vesicles.
Subsequent binding studies revealed that
Hp from specific granules binds to Hb.
Finally, the CCAAT enhancer binding protein-epsilon (C/EBP⑀) induced Hp transcription in a myeloid cell line, suggesting that Hp expression in myeloid cells,
as in hepatocytes, is at least partially
regulated by members of the C/EBP transcription factor family. Collectively, these
findings demonstrate that Hp is stored in
specific granules and is released by neutrophils in response to activation. Hence,
neutrophil-derived Hp might reduce tissue damage and bacterial growth at sites
of infection or injury by propagating antiinflammatory activities and Hb clearance.
(Blood. 2006;108:353-361)
© 2006 by The American Society of Hematology
Introduction
Infection and tissue injury initiate a local inflammatory response
and the subsequent release of proinflammatory cytokines, which
in turn mediate a systemic reaction. This so-called acute-phase
reaction (APR) is characterized by a marked increase in a
common set of plasma proteins known as acute-phase proteins
(APPs). APPs are predominantly synthesized in the liver and are
essential for reestablishing systemic homeostasis in response to
infection. APPs can be subcategorized according to their key
functions, such as protease inhibition, support of coagulation
and fibrinolysis, modulation of the immune response, and
clearance of toxic substances.1-3
Haptoglobin (Hp) is an APP protein whose plasma concentration increases several-fold in response to infection or injury. Hp
is synthesized as a single polypeptide chain and is proteolytically cleaved to a short ␣-chain and a long -chain that remain
connected through a disulfide bond.4 Humans differ from other
species by having 2 variants of the HP gene (denoted Hp 1 and
Hp 2) caused by partial gene duplication of the ␣-chain (␣1,
approximately 10 kDa; ␣2, approximately 18 kDa). Given that
both Hp variants contain a common -chain (approximately 39
kDa), humans have 3 major phenotypes of Hp, namely Hp 1-1
containing ␣1- molecules, Hp 2-2 containing ␣2- molecules,
and Hp 2-1 containing ␣1- and ␣2- molecules.5 The Hp 1-1
phenotype represents the basic structure of Hp, which is a dimer
of 2 ␣1- molecules joined by a single disulfide-bond between
the ␣1-chains. In contrast, the Hp 2-1 and Hp 2-2 phenotypes
generate various multimers of ␣1- and ␣2- molecules because
the ␣2-chains contain 2 cysteine residues (a result of gene
duplication) that form disulfide bonds with 2 other ␣-chains.5
The best understood function of Hp is to bind free hemoglobin (Hb) through the formation of high-affinity complexes.6,7
These Hp-Hb complexes (but not Hp or Hb alone) in turn bind
with high specificity to the CD163 scavenger receptor on
macrophages, resulting in endocytosis and subsequent intracellular degradation.8,9 Thus, Hp-Hb complex formation is thought
to reduce the loss of free Hb through glomerular filtration and to
support the recycling of iron.9,10 In addition, the immediate
clearance of Hb released from erythrocytes is crucial because
free Hb serves as a source of iron, which may otherwise enhance
bacterial growth and virulence.11,12 Finally, heme and iron
released from free Hb may participate in the generation of
reactive oxygen species (Fenton reaction) and may thus promote
From the Granulocyte Research Laboratory/Department of Hematology,
Rigshospitalet, the Laboratory for Gene Therapy Research/Department of
Clinical Biochemistry, Rigshospitalet, the Department of Hematology/Herlev
Hospital, and the 8th Rigshospitalet, University of Copenhagen, Copenhagen
Denmark; the Department of Medical Biochemistry, University of Aarhus,
Aarhus, Denmark; the Department of Hematology, Royal Manchester
Children’s Hospital, Manchester, United Kingdom; Booth Hall Children’s
Hospital, University of Manchester, Manchester, United Kingdom; the Division
of Hematology/Oncology, Cedars-Sinai Medical Center, Burns and Allan
Research Institute, David Geffen School of Medicine at University of California,
Los Angeles (UCLA); and the Department of Cell Biology, The Netherlands
Cancer Institute, Amsterdam, The Netherlands.
as Blood First Edition Paper, March 16, 2006; DOI 10.1182/blood-2005-09-3890.
Submitted September 29, 2005; accepted February 15, 2006. Prepublished online
© 2006 by The American Society of Hematology
BLOOD, 1 JULY 2006 䡠 VOLUME 108, NUMBER 1
Supported in part by the Danish Medical Research Council, the Danish Cancer
Research Foundation, the Novo Nordisk Foundation, the Amalie Jørgensens
Memorial Foundation, the Gangsted Foundation, and the Lundbeck
Foundation.
Reprints: Niels Borregaard, Department of Hematology-4042, Rigshospitalet,
University of Copenhagen, Blegdamsvej 9, 2100 Copenhagen-Ø, Denmark; email: borregaard@rh.dk.
The publication costs of this article were defrayed in part by page charge
payment. Therefore, and solely to indicate this fact, this article is hereby
marked ‘‘advertisement’’ in accordance with 18 U.S.C. section 1734.
353
From www.bloodjournal.org by guest on September 30, 2016. For personal use only.
354
BLOOD, 1 JULY 2006 䡠 VOLUME 108, NUMBER 1
THEILGAARD-MÖNCH et al
tissue injury.13,14 The latter has been substantiated in vivo by
demonstrating that Hp knockout mice experience severe Hbmediated tissue injury, particularly in the kidney, compared with
wild-type mice.15,16 Hence, Hp functions indirectly as a bacteriostatic agent and an antioxidant by facilitating the immediate
clearance of free Hb by macrophages.
A variety of other immunomodulatory effects have been
ascribed to Hp. It has been demonstrated that Hp binding to
activated neutrophils inhibits calcium influx and subsequent
generation of reactive oxygen species.17 In addition, it has been
reported that Hp suppresses macrophage functions such as
LPS-induced production of tumor necrosis factor alpha (TNF-␣),
proliferation and cytokine production by T cells, and proliferation of B cells.18-20 Hp has further been shown to bind to cells
through CD11b/CD18, CD22, and other yet undefined surface
antigens, suggesting that Hp exerts its immunomodulatory
effects through receptor-mediated signaling.20-23 Finally, it has
been reported that Hp suppresses heat- and oxidative stress–
induced misfolding and subsequent precipitation of a variety of
proteins. Hence, Hp has a genuine chaperone activity and might
thus reduce toxic effects associated with inappropriate aggregation of damaged (misfolded) extracellular proteins.24 Collectively, these findings indicate that Hp contributes to the reestablishment of homeostasis after local or systemic infection by
propagating various anti-inflammatory activities.
Polymorphonuclear neutrophilic granulocytes (neutrophils
[PMNs]) are key effector cells of innate immunity and of the
inflammatory response. Within minutes of injury, neutrophils
accumulate at sites of infection and initiate a first line of
defense.25-27 Neutrophils exert a broad range of defense mechanisms, including phagocytosis of microorganisms, release of
reactive oxygen species to phagosomes, and de novo synthesis
of chemokines and cytokines that recruit and modulate the
immune response of additional effector cells (neutrophils,
monocytes, T cells).25,27,28 Another defense mechanism, unique
to neutrophils, is the finely tuned release of prestored antimicrobial and proteolytic proteins to phagosomes and the extracellular environment. Antimicrobial and proteolytic proteins of
neutrophils are stored in distinct subsets of cytoplasmic granules, designated azurophil, specific, and gelatinase granules.25,27
A characteristic of granule formation is the sequential emergence of azurophil, specific, and gelatinase granules and their
constituent granule proteins during granulocyte differentiation,
namely in promyelocytes (PMs), myelocytes/metamyelocytes
(MYs), and bone marrow neutrophils (bm-PMNs), respectively.
These findings have fostered the targeting-by-timing theory,
stating that targeting of granule proteins to a distinct subset of
granules is determined by the time of their synthesis. Hence,
granule proteins stored in azurophil, specific, and gelatinase
granules are readily identified by their unique gene-expression
profiles during granulocyte differentiation.29-31 More recently,
Wagner et al32 reported that Hp is present, albeit not synthesized, in fully differentiated neutrophils, leading to the hypothesis that neutrophil-derived Hp is endocytosed from plasma.
Here, we provide evidence that neutrophil-derived Hp is
primarily synthesized in MYs during granulocyte differentiation,
stored in specific granules of fully differentiated neutrophils, and
exocytosed immediately in response to activation. These findings
implicate that neutrophils release Hp at sites of infection or injury
to protect tissue from damage and to reduce bacterial growth during
the initial phase of the immune response.
Materials and methods
Isolation of peripheral-blood neutrophils and
bone marrow populations
Blood samples, buffy coats, and bone marrow aspirates were obtained from
healthy donors and a child with confirmed specific granule deficiency
(SGD) after informed consent had been given, in accordance with the local
ethics committees of the cities of Copenhagen, Frederiksberg, and Manchester.33 Peripheral-blood neutrophils (pb-PMNs) were isolated from blood
samples or buffy coats by density centrifugation and subsequent hypotonic
lysis of erythrocytes, as described previously.34 Populations highly enriched
in promyelocytes (PMs), myelocytes/metamyelocytes (MYs), and bone
marrow neutrophils (bm-PMNs) were isolated from BM aspirates by
2-layer density centrifugation and subsequent immunomagnetic depletion
of nongranulocytic cells, as described previously.31,35 All steps of immunomagnetic sorting were performed immediately after cell collection at 4°C or
less with the use of nonpyrogenic reagents and plasticware to minimize
cellular activation.
Cytospins were prepared by centrifugation of 1 to 2 ⫻ 105 isolated cells
onto glass slides (300 rpm, 10 minutes, room temperature [RT]; centrifuge
used was a Shandon Cytocentrifuge [Thermo Electron, Waltham, MA]).
The purity of isolated BM and neutrophil populations was assessed by
microscopy of Wright-Giemsa–stained cytospins. Cell numbers were
assessed with an improved Neubauer hemocytometer.
Immunocytochemistry
Cytospins of purified BM and neutrophil populations were fixed in TBS
(50 mM Tris, 150 mM NaCl, pH 7.6)/4% formaldehyde (37% stock; Sigma,
St Louis, MO) at RT for 20 minutes, washed in TBS, and permeabilized in
TBS/1% Triton X-100 (Sigma) at RT for 30 minutes. Cytospins were then
washed in TBS/1% BSA, and nonspecific binding was blocked by
incubation in TBS/1% BSA (Sigma) at RT for 30 minutes. Then cytospins
were probed at RT for 30 minutes with the following primary antibodies
diluted in TBS/0.25% BSA: rabbit anti–human haptoglobin (1:1000; A
0030; DakoCytomation, Glostrup, Denmark), rabbit anti–human lactoferrin
(1:1000; gift from DakoCytomation), and control rabbit IgG (1:500; X
0936; DakoCytomation). Cytospins were washed twice in TBS, incubated
at RT for 30 minutes with alkaline phosphatase–conjugated anti–rabbit/
mouse polymer, washed twice in TBS, and stained with Fast-Red as
recommended by the manufacturer (Dual Envision System-AP; DakoCytomation). Finally, cytospins were washed in running tap water for 10 minutes, counterstained for 1 minute in Mayer hematoxylin, washed again in
running tap water for 3 minutes, and mounted. Cytospins were examined
under a BX51 microscope equipped with a DP70 photosystem with analy
SIS 5.0 software (Olympus, Hamburg, Germany) and a 40⫻/0.85 numeric
aperture oil objective. PowerPoint software (Microsoft, Redmond, WA)
was used to prepare the images.
Exocytosis studies
Neutrophils were isolated from freshly collected blood samples or buffy
coats and resuspended at a density of 3 ⫻ 107 cells/mL in Krebs-Ringer
phosphate buffer with glucose (KRG; 130 mM NaCl, 5 mM KCl, 1.27 mM
MgSO4, 0.95 mM CaCl2, 10 mM NaH2PO4/Na2HPO4, 5 mM glucose, pH
7.4). To stimulate exocytosis of granule proteins by neutrophils, 1 mL cell
suspension was preincubated for 5 minutes at 37°C and subsequently
stimulated for 15 or 30 minutes at 37°C by the addition of the following
agents: ionomycin (1 M; Calbiochem, La Jolla, CA), phorbol-12myristate 13-acetate (PMA; 2.5 g/mL; Sigma), formyl-Met-Leu-Phe
(fMLP; 100 nM; Sigma), TNF-␣ (50 ng/mL; Sigma), and 10% autologous
serum-opsonized Escherichia coli (multiplicity of infection, 10 bacteria/
cell). Opsonized E coli bacteria (XL-1 Blue; Stratagene, La Jolla, CA) were
prepared using fresh autologous serum essentially as described previously.36 Control cells were incubated on ice for 20 or 35 minutes.
Stimulation was stopped with the addition of 1 mL ice-cold KRG to cell
suspensions. Cells were immediately pelleted, and the cell pellets and
From www.bloodjournal.org by guest on September 30, 2016. For personal use only.
BLOOD, 1 JULY 2006 䡠 VOLUME 108, NUMBER 1
supernatants containing exocytosed granule were subjected to enzymelinked immunosorbent assay (ELISA), as described previously, or were
resuspended in 2 ⫻ Laemmli buffer for subsequent Western blot analysis.37
Immunoelectron microscopy
Isolated blood neutrophils were fixed for 24 hours in 0.1 M PHEM buffer
(240 mM PIPES, 100 mM HEPES, 8 mM MgCl2, 40 mM EGTA, pH 6.9)
containing 4% paraformaldehyde, as described previously.38 Ultrathin
cryosections of neutrophils were prepared and incubated at RT with the
primary antibodies rabbit anti–human haptoglobin (DakoCytomation) and
rabbit anti–human lactoferrin (Cappel Laboratories, Cochranville, PA) and
10 nm or 15 nm protein A gold, as described previously.38 Controls were
labeled with irrelevant rabbit antiserum. After immunolabeling, the cryosections were embedded in a mixture of methylcellulose and uranyl acetate and
were examined through an electron microscope (CM 10; Philips, Eindhoven, The Netherlands).
Subcellular fractionation
Neutrophils isolated from buffy coats were incubated in saline/5 mM
diisopropylfluorophosphate (DFP; Aldrich Chemical, Milwaukee, WI) for
5 minutes, pelleted (200g, 6 minutes, 4°C), and resuspended at 3 ⫻ 107
cells/mL in disruption buffer (100 mM KCl, 3 mM NaCl, 1 mM Na2 ATP,
3.5 mM MgCl2, 10 mM PIPES, pH 7.2) containing 0.5 mM phenylmethylsulfonyl fluoride (PMSF; Sigma). Cells were disrupted by nitrogen
cavitation at 600 psi.39 Nuclei and intact cells were pelleted (400g,
15 minutes). Postnuclear supernatant (S1; 10 L) was carefully applied on
top of a Percoll gradient including 3 layers of 9 mL Percoll with densities of
1.05, 1.09, and 1.12 g/mL. Gradients were generated by adding precalculated amounts of Percoll (range, 1.129-1.131 g/mL; Amersham Bioscience
AB, Uppsala, Sweden) to disruption buffer/0.5 mM PMSF (Sigma) to
obtain densities of 1.05, 1.09, and 1.12 g/mL.39 The 3-layer gradient topped
by the postnuclear supernatant (S1) was centrifuged at 37 000g for
30 minutes for subcellular fractionation. This resulted in 4 major bands—
the ␣-band enriched in azurophil granules, the 1-band enriched in specific
granules, the 2-band enriched in gelatinase granules, and the ␥-band
enriched in cell membranes and secretory vesicles containing plasma
proteins. Fractions of 1 mL were aspirated from the bottom of the 3-layer
gradient, and 450 L from each fraction was centrifuged for 20 minutes at
28 psi in an airfuge (Beckman, Palo Alto, CA) to sediment the Percoll. The
supernatant containing the cellular material was resuspended in phosphatebuffered saline (PBS) and subjected to ELISA, as described previously, or
was mixed with an equal volume of 2 ⫻ Laemmli buffer for subsequent
Western blot analysis.37
N-glycosidase F treatment
Pooled subcellular -fractions containing Hp from specific granules
(20 L) and purified plasma Hp (10 L; Sigma) were deglycosylated by
peptide N-glycosidase F (PNGase-F) treatment for 3 hours at 37°C in
accordance with the manufacturer’s instructions (New England Biolabs,
Beverly, MA). After deglycosylation, samples were mixed with equal
volumes of 2 ⫻ Laemmli buffer for Western blot analysis.
Hp-Hb affinity precipitation
Human HbA0 (Sigma) was coupled to CNBr-activated Sepharose 4B beads
according to the manufacturer’s instructions (Amersham Biosciences).
HbA0 Sepharose beads and control Sepharose beads were washed twice in
PBS (pH 7.8) and incubated with pooled subcellular -fractions resuspended in PBS overnight at 4°C. After incubation, samples were washed
6 ⫻ in PBS (2800g, 2 minutes, RT), and Hp was eluted in SDS containing
sample buffer (0.02 M Tris, pH 6.8, 5% SDS, 17.4% glycerol) for
subsequent Western blot analysis.
Surface plasmon resonance analysis
Binding studies of neutrophil-derived Hp to Hb were performed by surface
plasmon resonance (SPR) analysis. To obtain neutrophil-derived Hp
Hp IS A SPECIFIC GRANULE PROTEIN OF NEUTROPHILS
355
contained in specific granules, subcellular -fractions were prepared from
pooled buffy coats (including Hp phenotypes 1-1, 2-1, 2-2), and Hp was
highly enriched by affinity chromatography with a column containing 5 mg
rabbit anti–human haptoglobin (DakoCytomation) immobilized on 4 mL
CNBr-activated Sepharose beads (Amersham Bioscience).
SPR analysis was performed essentially as described previously with a
BIAcore 3000 instrument (BIAcore AB, Uppsala, Sweden) and BIAcore
CM5 sensor chips containing immobilized human HbA0 (Sigma) corresponding to 67 to 83 fmol/mm2 protein.8,40 Sensograms were generated using
samples of purified neutrophil-derived Hp and plasma Hp (Sigma). Binding
data were analyzed using the BIAmolecular analysis evaluation program
(version 3.1; BIAcore AB).40
Western blot analysis
Samples were diluted with an equal volume of 2 ⫻ Laemmli buffer and
were denatured at 100°C for 10 minutes.41 Samples were then electrophoresed on 10% and 12% SDS polyacrylamide gels (BDH Laboratory
Supplies, Poole, United Kingdom) or 4% to 16% gradient SDS polyacrylamide gels and were transferred to nitrocellulose membranes (Amersham
Bioscience) by electroblotting. Membranes were then incubated as indicated with primary rabbit anti–human haptoglobin (1:1000; DakoCytomation), myeloperoxidase (MPO; 1:1000, A0398; DakoCytomation), and
lactoferrin (1:10 000; gift from DakoCytomation) followed by a secondary
horseradish peroxidase–conjugated swine antirabbit antibody (1:1000,
P0217; DakoCytomation) or an alkaline phosphatase–conjugated goat
antirabbit antibody (1:1000, D0487; DakoCytomation). Binding of antibodies was visualized by enhanced chemiluminescence (ECL; Amersham
Bioscience) or a mixture of nitroblue tetrazolium (NBT) and 5-bromo-4chloro-3-indolyl-1-phosphate (BCIP; Promega, Madison, WI).
32Dcl3 cells
The murine 32Dcl3 cell line, generated from diploid myeloid progenitors,
proliferates in the presence of IL-3 and can differentiate into mature
neutrophils within 10 to 12 days in response to granulocyte–colonystimulating factor (G-CSF) stimulation.42 32Dcl3 cells were transduced
with a retroviral vector constitutively expressing the C/EBP⑀-ER fusion
protein, which is maintained in the cytoplasm and only translocates to the
nucleus to exert C/EBP⑀ activity in the presence of the estrogen derivative
4-hydroxy-tamoxifen (4-HT).
32Dcl3 cells were maintained in Iscove modified Dulbecco medium
(IMDM; Invitrogen, San Diego, CA) containing 10% heat-inactivated calf
serum, 1 ng/mL murine IL-3 (StemCell Technologies, Vancouver, BC,
Canada), and 100 U/mL penicillin combined with 100 g/mL streptomycin
(Invitrogen).
The C/EBP⑀-ER cDNA construct was prepared by linking the fulllength human C/EBP⑀ cDNA in-frame to a modified tamoxifen-responsive
estrogen receptor hormone-binding domain (murine ER; amino acids
281-599).43 The C/EBP⑀-ER and ER cDNA were inserted into the
polylinker of the pBabePuro retroviral vector (Nolan Laboratory home
page: http://www.stanford.edu/group/nolan/index.html). The pBabePuro-C/
EBP⑀-ER vector and the pBabePuro-ER control vector were transfected
into the ecotropic packaging cell line Phoenix NX by calcium-phosphate
precipitation. After 24 hours, 32Dcl3 cells were cocultured with transfected
Phoenix cells for another 48 hours in 32Dcl3 medium plus 4 g/mL
polybrene Sigma. Subsequently, 32Dcl3 cells were selected in puromycin
(2 g/mL), and subclones were generated by transfer of single cells into
96-well dishes using an automated Quickcell transfer device (Stoelting,
Wood Dale, IL).34
To define whether C/EBP⑀ can transactivate Hp expression in myeloid
progenitors, C/EBP⑀ activity was induced in 32Dcl3-C/EBP⑀-ER cells by
the addition of 200 nM 4-HT (Sigma) to the medium. 32Dcl3-ER cells
induced by 4-HT served as control. Total RNA was isolated from cells using
TRIzol (Invitrogen) before and after 4-HT induction at indicated time
points. Subsequently, Hp expression was assayed by real-time RT-PCR.
From www.bloodjournal.org by guest on September 30, 2016. For personal use only.
356
THEILGAARD-MÖNCH et al
BLOOD, 1 JULY 2006 䡠 VOLUME 108, NUMBER 1
Real-time RT-PCR
Expression of Hp transcripts in 32Dcl3-C/EBP⑀-ER and 32Dcl3-ER cells
were assessed as described previously.44 Briefly, first-strand cDNA was
generated by reverse transcription of 5 g total RNA at 42°C for 1 hour
with the use of a T7-oligo(dT)24 primer and Superscript II, as described by
the manufacturer (Invitrogen). First-strand cDNA was then subjected to
real-time PCR using the following primers and probes: -actin forward
primer, GAA GTC CCT CAC CCT CCC A; -actin reverse primer, GGC
ATG GAC GCG ACC A; -actin probe, AAG CCA CCC CCA CTC CTA
AGA GGA GG; Hp forward primer, TTC AGG GCT CAC TAG AAG GCT
G; Hp reverse primer, TTC CCC CTC TTC CAT GGC; Hp probe, ACA
TGG CAG GGC AGG CTG GG. The constitutively expressed housekeeping gene -actin was used to normalize Hp expression.
Results
Hp is synthesized during granulocyte differentiation
The hallmark of granulocyte differentiation is the sequential
formation of azurophil, specific, and gelatinase granules and
their constituent granule proteins. Accordingly, granule proteins
are readily identified by their unique gene-expression profiles
during granulocyte differentiation.29-31 We recently characterized the transcriptional program of terminal granulocyte differentiation by comprehensive microarray analysis of populations
highly enriched in promyelocytes (PMs), myelocytes/metamyelocytes (MYs), bone marrow neutrophils (bm-PMNs), and
peripheral-blood neutrophils (pb-PMNs).31 By hierarchic clustering of differentially regulated genes in our data set, we identified
clusters of genes whose expression profiles were identical to
those of defined azurophil, specific, and gelatinase granule
proteins. This analysis demonstrated that Hp was annotated to
the gene cluster encompassing various specific granule proteins
including lactoferrin (Lf), a designated marker protein of
specific granules. Hence, Hp transcripts were highly expressed
in MYs (Figure 1A). Immunocytochemistry revealed that Hp
protein, like Lf, is detectable in the cytosol of populations
highly enriched in MYs, bm-PMNs, and pb-PMNs, but not PMs
(Figure 1B). Overall, these findings demonstrate that Hp is
synthesized in MYs and remains stored in a cytosolic compartment throughout granulocyte differentiation.
Hp and Lf colocalize in specific granules of neutrophils
To investigate the subcellular localization of Hp in neutrophils,
we isolated subcellular fractions from disrupted neutrophils by
3-layer density gradient centrifugation. With this method,
fractions highly enriched in azurophil, specific, and gelatinase
granules and secretory vesicles are readily identified based on
their high content of distinct marker proteins, including myeloperoxidase (MPO), gelatinase (Gel), and plasma albumin (Alb),
respectively (Figure 2). As depicted in Figure 2, Western blot
analysis demonstrated that the major part of Hp colocalized in
subcellular fractions with a high content of Lf, a marker protein
of specific granules. In contrast, subcellular fractions with high
contents of MPO (azurophil granules), Gel (gelatinase granules), and Alb (secretory vesicles) contained only low amounts
of Hp (Figure 2). To further substantiate the colocalization of Hp
and Lf in specific granules of neutrophils, we performed
immunoelectron microscopy on intact neutrophils using antibodies raised against Hp and Lf. As shown in Figure 3, Hp and Lf
indeed colocalized in electron-dense specific granules. Hp was
not observed in organelles other than those containing Lf. Taken
Figure 1. Hp and lactoferrin are synthesized at the myelocyte stage and stored
in the cytosol of cells throughout granulocyte differentiation. BM populations
highly enriched in PMs, MYs, bm-PMNs, and pb-PMNs were isolated from healthy
donors. (A) Total RNA was purified from BM and PB populations and subjected to
microarray analysis to monitor the expression profiles for Hp and Lf (marker for
specific granules) transcripts during granulocyte differentiation (mean ⫾ SD; n ⫽ 3).31
(B) Immunocytochemical staining of BM and PB populations using rabbit anti–human
Hp and Lf antibodies demonstrates the cytoplasmic localization (red) of Hp and Lf
from the myelocyte stage throughout granulocyte differentiation. BM populations
highly enriched in PMs stained with rabbit anti–human Hp or Lf antibodies and
populations stained with irrelevant rabbit IgG antibodies were all negative. Original
magnification, ⫻ 400.
together, these findings indicate that Hp is a genuine specific
granule protein.
Neutrophils release Hp in response to activation
Once neutrophils have been attracted to sites of infection or injury,
they respond to various inflammatory stimuli by immediate exocytosis of effector proteins primarily stored in granules and secretory
vesicles. Exocytosis of effector proteins can be mimicked by in
vitro activation of neutrophils using various stimuli. Several
studies by our laboratory have documented that neutrophils stimulated by phorbol myristate acetate (PMA) release proteins contained in specific/gelatinase granules and secretory vesicles but
only minor amounts of proteins contained in azurophil granules,
whereas neutrophils stimulated by the calcium ionophore ionomycin release proteins stored in all 4 organelles.37,45 In addition,
inflammatory stimuli such as fMLP, TNF-␣, and serum-opsonized
bacteria have been shown to activate neutrophils and to induce
exocytosis of granule proteins in vitro.37,46
To investigate whether stimuli affecting the activation of
neutrophils also affected the release of Hp, purified neutrophils
were stimulated by PMA, ionomycin, fMLP, TNF-␣, and serumopsonized bacteria. Subsequent Western blot analysis of supernatants collected from PMA and ionomycin-stimulated neutrophils
revealed a marked corelease of Hp and Lf (Figure 4A). Consistent
From www.bloodjournal.org by guest on September 30, 2016. For personal use only.
BLOOD, 1 JULY 2006 䡠 VOLUME 108, NUMBER 1
Hp IS A SPECIFIC GRANULE PROTEIN OF NEUTROPHILS
357
tions and plasma, resulting in -chain bands with identical
molecular weights of 30 kDa (Figure 5A).
Taken together, these findings demonstrate that neutrophils
contain 2 forms of Hp, a scarce form of low molecular weight,
endocytosed from plasma and stored in secretory vesicles, and a
previously undescribed abundant form of high molecular weight
that is synthesized in MYs and stored in specific granules.
Neutrophil-derived Hp stored in specific granules binds to Hb
Figure 2. Highly glycosylated Hp and lactoferrin colocalize in subcellular
fractions enriched in specific granules. Subcellular fractions were isolated from
purified peripheral-blood neutrophils and assayed by ELISA and Western blot
analysis. Lines indicate subcellular fractions containing relative concentrations for
MPO (marker for azurophil granules, peak concentration in fraction 5-433 g/mL), Lf
(marker for specific granules, peak concentration fraction 11-187 g/mL), Gel
(marker for gelatinase granules, peak concentration in fraction 16-33 g/mL), and Alb
(marker for secretory vesicles, peak concentration in fraction 21-2.2 g/mL). Two
forms of Hp were detected by Western blot analysis, an abundant form with a highly
glycosylated -chain of approximately 45 to 65 kDa that colocalized in fractions with
high content of the specific granule protein Lf (fractions 10-15) and a scarce form with
a -chain of 39 kDa that colocalized in fractions containing Alb (fractions19-23), a
protein endocytosed from plasma and stored in secretory vesicles of neutrophils.
with these findings, ELISA and Western blot analyses of supernatants and cell pellets collected after the stimulation of neutrophils
by fMLP, TNF-␣, and serum-opsonized bacteria revealed marked
corelease of Hp, Lf, and gelatinase but not of MPO (Figure 4B). In
contrast, unstimulated neutrophils did not release Hp, Lf, or
gelatinase (Figure 4A-B).
Overall, these observations substantiate the colocalization of
Hp and Lf in specific granules of neutrophils.
Neutrophil-derived Hp stored in specific granules is more
glycosylated than plasma-derived Hp
Humans have 2 variants of the HP gene, the Hp 1 variant with a
short ␣1-chain (approximately 10 kDa) and the Hp 2 variant with a
long ␣2-chain (approximately 18 kDa), resulting from partial gene
duplication of the ␣-chain. Both Hp variants have a common
-chain (approximately 39 kDa) that contains 4 N-linked glycans.
In the present study, we identified 2 forms of Hp in neutrophils: (1)
an abundant form with a -chain of high molecular weight
(approximately 45-65 kDa) that colocalized in subcellular fractions
with a high content of Lf (Figure 2, fractions 10-15; Figure 5A,
-fractions) and (2) a scarce form with a -chain of low molecular
weight (approximately 39 kDa) that primarily colocalized in
subcellular fractions with a high content of albumin, a plasma
protein that is endocytosed and stored in secretory vesicles (Figure
2, fractions 19-23; Figure 5A, ␥ fractions).
Consistent with these findings, Western blot analysis of nonreduced samples, obtained from patients with the Hp 1-1 and Hp 2-1
phenotypes, revealed that Hp dimers and Hp multimers contained
in pooled subcellular -fractions—ie, specific granules—have
higher molecular weights than those contained in plasma
(Figure 5B).
To investigate whether the higher molecular weight of neutrophilderived Hp compared with plasma-derived Hp was the result of a
higher degree of -chain glycosylation, pooled subcellular -fractions (-chain, approximately 45-65 kDa) and purified plasma Hp
(-chain, approximately 39 kDa) were subjected to N-deglycosylation by PNGase-F. Western blot analysis demonstrated a complete
deglycosylation of -chains contained in pooled subcellular -frac-
It is well established that plasma-derived Hp can bind Hb and
facilitate its clearance by macrophages. To investigate whether the
highly glycosylated form of Hp contained in specific granules of
neutrophils is capable of binding Hb, we performed affinity
precipitation and surface plasmon resonance (SPR) analysis.
Affinity precipitation using pooled subcellular -fractions and
Hb-Sepharose beads resulted in precipitation of the highly glycosylated neutrophil-derived form of Hp, indicating tight binding to Hb
(Figure 6A). Subsequently, SPR analysis revealed similar binding
curves and kinetics for neutrophil- and plasma-derived Hp to Hb,
indicating a similar functional affinity (Figure 6B). Because the
neutrophil-derived Hp was prepared from pooled buffy coats
(including Hp phenotypes 1-1, 2-1, 2-2) and therefore contained
various multimeric Hp forms, an exact affinity constant could not
be calculated from the binding curve. However, the estimated
dissociation rate constants (Kd granula, 3.9 ⫻ 10⫺4 s⫺1; Kd plasma,
1.7 ⫻ 10⫺4 s⫺1) were similar, indicating that neutrophil-derived Hp
binds to Hb with a tightness similar to that of plasma-derived Hp.
Blood cells from a patient with SGD lack neutrophil-derived Hp
SGD is a rare congenital disorder (5 reported cases) characterized
by the lack of specific and gelatinase granules and their constituent
granule proteins in neutrophils. Neutrophils from patients with
SGD display atypical bilobed nuclei and are defective in chemotaxis and antibacterial activity. As a result, SGD patients are
severely immunocompromised and experience early and frequent
bacterial infections of the skin and respiratory tract.47 Because
SGD neutrophils lack specific granule proteins, such as Lf, but
contain azurophil granule proteins, such as MPO, we reasoned that
Hp of high molecular weight (-chain, approximately 45-65 kDa)
should not be detected in blood cells from SGD patients. To test this
hypothesis, we obtained blood-cell lysates from a child with a
diagnosis of SGD.33 Immediately after birth, the child presented
Figure 3. Hp and lactoferrin colocalize in specific granules of neutrophils.
Cryosections of peripheral-blood neutrophils were labeled with rabbit anti–human Hp
antibodies followed by a 10-nm protein A–gold probe (left). In addition, cryosections
(right) were double-labeled, first with a rabbit anti–human Hp antibody and a 10-nm
protein A–gold probe and subsequently with a rabbit anti–human Lf antibody and a
15-nm protein A–gold probe. Immunoelectron microscopy demonstrates the ultrastructural colocalization of Hp (arrows) and Lf in specific granules.
From www.bloodjournal.org by guest on September 30, 2016. For personal use only.
358
THEILGAARD-MÖNCH et al
BLOOD, 1 JULY 2006 䡠 VOLUME 108, NUMBER 1
Figure 4. Neutrophils release Hp in response to activation. (A)
Purified peripheral-blood neutrophils were stimulated by the calcium ionophore ionomycin (1 M) and PMA (2.5 g/mL) for 15
minutes, and the contents of exocytosed Hp and Lf in the
supernatant were detected by Western blot analysis. Ionomycinand PMA-activated neutrophils released Hp and Lf, whereas
unstimulated neutrophils did not. (B) Purified neutrophils from one
donor were stimulated for 15 or 30 minutes by PMA (2.5 g/mL),
fMLP (100 nM), TNF-␣ (50 ng/mL), and 10% autologous serum–
opsonized E coli (multiplicity of infection, 10 bacteria per cell).
Supernatants containing exocytosed granule proteins and cell
pellets were subjected to ELISA analysis (MPO, Lf, Gel, top
histogram) or Western blot analysis (Hp, lower histogram). The
percentage of MPO, Lf, and Gel released by neutrophils was
calculated as the amount of protein detected in the supernatant
divided by the total amount of protein detected in the supernatant
and pellet multiplied by 100.
with postnatal sepsis and delayed separation of the umbilical
cord. These incidents were succeeded by recurrent opportunistic
infections requiring frequent courses of intravenous broadspectrum antibiotic treatment. Peripheral-blood smears demonstrated neutrophils with abnormal morphology, including nuclei
with Pelger-Huet anomaly (bilobed rather than trilobed appearance), and electron microscopy demonstrated azurophil but not
specific granules in blood neutrophils.33
Blood samples obtained from the SGD patient and a healthy
donor were depleted of erythrocytes, and lysates, including total
nuclear cells (neutrophils and mononuclear cells) and residual
plasma were subjected to Western blot analysis with the use of
anti–human Hp, Lf, and MPO antibodies. Hp of high molecular
weight (-chain, approximately 45-65 kDa) and Lf were detected
in cell lysates prepared from the healthy donor, but neither protein
was detected in cell lysates from the patient with SGD (Figure 7).
In contrast, comparable amounts of the azurophil granule protein
MPO were detected in SGD and control lysates (Figure 7). Because
of plasma contamination, Hp of low molecular weight (-chain,
approximately 39 kDa) was detected in SGD and control lysates.
These results substantiate our findings that Hp is a genuine specific
granule protein and are consistent with previous reports indicating
that patients with SGD have a selected defect in the transcriptional
regulation of granule protein expression in myeloid cells (but
apparently not other cell types).
Induction of C/EBP-epsilon increases Hp transcript levels in
myeloid 32Dcl3 cells
Hp expression in the liver is partially regulated by C/EBP.48,49
Another member of the C/EBP transcription factor family, C/EBP⑀,
has been defined as a key regulator of terminal granulocyte
differentiation (transition from promyelocytes into mature neutrophils).50 Functional loss of C/EBP⑀ in mice, and some but not all
currently reported SGD patients, results in the generation of
Figure 5. Hp located in specific granules of neutrophils is more glycosylated
than plasma-derived Hp. (A) Pooled subcellular fractions isolated from peripheralblood neutrophils and purified plasma Hp were analyzed by Western blot analysis
using rabbit anti–human Hp antibody. Western blots to the left demonstrate pooled
subcellular fractions highly enriched in azurophil granule proteins (␣-fraction),
specific granule protein (1-fraction), gelatinase granule proteins (2-fractions), and
secretory vesicles containing mainly plasma proteins (␥-fractions). These Western
blots show that the -chain of Hp, primarily present in 1- and 2-fractions, has a
higher molecular weight (approximately 45-65 kDa) than the -chain of plasmaderived Hp (39 kDa) primarily present in ␥-fractions (secretory vesicles). Western
blots to the right demonstrate that Hp -chains from specific granules (pooled
-fractions) and plasma samples have an identical molecular weight of 30 kDa after
complete N-deglycosylation by PNGase-F. (B) Western blot analysis of nonreduced
pooled -fractions and plasma samples, prepared from patients with the Hp 1-1 and
Hp 2-1 phenotypes with the use of rabbit anti–human Hp antibody. These Western
blots demonstrate that Hp dimer and Hp multimers contained in pooled subcellular
-fractions (ie, specific granules) have higher molecular weights than those contained in plasma.
Figure 6. Neutrophil-derived Hp stored in specific granules binds to Hb. (A)
Nonreduced pooled subcellular -fractions from neutrophils were incubated with
Hb-Sepharose beads and Sepharose control beads. Subsequently, beads were
precipitated, and Hb, control eluates (contr. eluate), and pooled subcellular -fractions (granula control [gran. contr.]) were subjected to Western blot analysis using
rabbit anti–human Hp antibody. The Western blot demonstrates the presence of
highly glycosylated neutrophil-derived Hp in eluates from Hb-Sepharose beads but
not from Sepharose control beads, indicating that Hp contained in specific granules
binds to Hb. (B) SPR analysis demonstrating the binding curve of highly glycosylated
neutrophil-derived (granula) Hp and plasma-derived Hp to a Hb BIAcore sensor chip.
Response units (RUs) correspond to the response difference between Hp and control
samples. SPR analysis demonstrates similar binding curves/kinetics and dissociation
rate constants (Kd granula, 3.9 ⫻ 10⫺4 s⫺1; Kd plasma, 1.7 ⫻ 10⫺4 s⫺1) for both types
of Hp.
From www.bloodjournal.org by guest on September 30, 2016. For personal use only.
BLOOD, 1 JULY 2006 䡠 VOLUME 108, NUMBER 1
Figure 7. Hp and lactoferrin are absent in blood cells from a patient with SGD.
Blood samples from a patient with SGD and a healthy donor (control) were depleted
of erythrocytes, and lysates including total nuclear cells (neutrophils and mononuclear cells) and residual plasma were subjected to Western blot analyses using
rabbit anti–human MPO, Lf, and Hp antibodies. Western blot analyses demonstrated
that blood cells from the SGD patient expressed the azurophil granule protein MPO at
comparable levels but expressed neither the specific granule protein Lf nor the
highly-glycosylated Hp -chain with a molecular weight of 45 to 65 kDa. Contamination of cell lysates with residual plasma resulted in the detection of Hp -chain with a
low molecular weight (39 kDa) in the control sample and the sample obtained from
the SGD patient.
dysfunctional neutrophils lacking specific and gelatinase granule
proteins.47,51,52 Consistent with these findings, we have demonstrated that C/EBP⑀ transcripts and protein are highly expressed in
MYs (but not PMs or bm-PMNs) when specific granule proteins,
including Hp and Lf, are synthesized.31,53 In addition, C/EBP⑀ has
been shown to induce expression of the specific granule protein Lf
in vitro.54 Taken together, these findings suggest that C/EBP⑀ might
contribute to the transcriptional regulation of Hp expression in
myeloid cells.
To investigate whether C/EBP⑀ can induce the expression of Hp
in vitro, we generated a myeloid 32Dcl3 cell line constitutively
expressing a fusion protein containing the C/EBP⑀ wild-type
protein and the ligand-binding domain of the estrogen receptor
(32Dcl3-C/EBP⑀-ER). With this cell line, the C/EBP⑀-ER fusion
protein is maintained in the cytoplasm and only translocates to the
nucleus to exert C/EBP⑀ activity in the presence of the estrogen
derivative 4-HT. Hence, the transcriptional activity of C/EBP⑀ can
be analyzed in an inducible manner. A 32Dcl3 cell line expressing
only the ligand-binding domain of the estrogen receptor served as
control (32Dcl3-ER).
Induction of 32Dcl3-C/EBP⑀-ER cells by 4-HT resulted in a
3.8-fold increase of Hp transcript levels within 1 day; after 4 days
of 4-HT induction, Hp transcript levels were maximally increased
(7.3-fold higher than baseline; Figure 8). Induction of 32Dcl3-ER
control cells by 4-HT did not result in any increase of Hp transcript
levels compared with baseline (0.2- to 1.6-fold change). These
findings indicate that the expression of Hp in myeloid cells, as in
hepatocytes, is at least partially regulated by members of the
C/EBP family.
Hp IS A SPECIFIC GRANULE PROTEIN OF NEUTROPHILS
359
demonstrated the colocalization of Hp and Lf in specific granules, a
finding consistent with the absence of Hp and Lf in blood cells
collected from a patient with SGD. Finally, functional studies
revealed that neutrophils corelease Hp and Lf, but not MPO, in
response to PMA and various inflammatory stimuli that induce
exocytosis of proteins primarily stored in secretory vesicles and
specific/gelatinase granules, but not in azurophil granules.
Although immunoelectron microscopy and exocytosis assays
are excellent tools to assess the subcellular localization of proteins
in azurophil compared with specific/gelatinase granules, their
levels of resolution might be insufficient to distinguish whether
proteins are localized primarily in specific or gelatinase granules.
Accordingly, more subtle assays, including subcellular fractionation and gene-expression profiling, are the methods of choice to
subcategorize specific and gelatinase granule proteins of neutrophils. In the present study, subcellular fractionation showed that Hp
colocalized with Lf, not gelatinase. In addition, gene-expression
profiling demonstrated that transcripts for Hp and Lf were expressed at significantly lower levels in bm-PMNs compared with
MYs and were barely detectable in pb-PMNs. In contrast, transcripts for gelatinase were expressed at the highest levels in MYs
and bm-PMNs and at high levels in pb-PMNs (data not shown).31
Hence, Hp synthesis is terminated simultaneously with that of Lf
during granulocyte differentiation and earlier than that of gelatinase. Based on these criteria, Hp is subcategorized as a specific
rather than a gelatinase granule protein.
Hp is a highly glycosylated protein whose -chains contain 4
N-linked glycans. Of interest, neutrophils contain 2 forms of Hp, a
hitherto undescribed abundant form with highly glycosylated
-chains (45-65 kDa) that is stored in specific granules and a scarce
form with “normally” glycosylated -chains (39 kDa) that is stored
in secretory vesicles and thus is endocytosed from plasma. Wagner
et al32 recently reported that fully differentiated pb-PMNs store but
do not synthesize Hp, leading to the hypothesis that neutrophilderived Hp is endocytosed from the plasma and thus is primarily of
liver origin. Results presented here are consistent with the presence
of scarce amounts of liver-derived Hp endocytosed from plasma
and stored in secretory vesicles of pb-PMNs. However, the results
presented here extend earlier findings substantially by demonstrating that the major part of Hp present in fully differentiated
pb-PMNs is not of liver origin but rather is synthesized in MYs and
stored in specific granules. Affinity precipitation and SPR analysis
demonstrated that highly glycosylated Hp from specific granules
binds to Hb with a tightness similar to that of plasma-derived Hp.
These findings support the idea that the glycosylation pattern of
-chains is characteristic for the cellular origin of Hp and does not
Discussion
The present study provides evidence that Hp is a genuine specific
granule protein of human neutrophils by several means. First, Hp
transcripts were highly expressed in MYs during granulocyte
differentiation. In agreement with this finding, immunocytochemistry demonstrated the presence of Hp protein in cells from the MY
stage and throughout granulocyte differentiation. In addition,
immunoelectron microscopy and subcellular fractionation studies
Figure 8. Hp expression in myeloid 32Dcl3 cells is induced by C/EBP⑀. Myeloid
32Dcl3 cells constitutively expressing a fusion protein containing the C/EBP⑀
wild-type and the ligand-binding domain of the estrogen receptor (32Dcl3-C/EBP⑀ER) were induced with 4-HT, resulting in nuclear translocation of C/EBP⑀-ER. Hp
mRNA levels were measured relative to -actin levels by reverse transcription (RT)
real-time PCR at the indicated time points. Changes of Hp mRNA levels at indicated time
points after 4-HT induction were calculated relative to the Hp mRNA level before 4-HT
induction (0 hour) (mean ⫾ SD; n ⫽ 3). Myeloid 32Dcl3 cells constitutively expressing the
ligand-binding domain of the estrogen receptor (32Dcl3-ER) served as control.
From www.bloodjournal.org by guest on September 30, 2016. For personal use only.
360
BLOOD, 1 JULY 2006 䡠 VOLUME 108, NUMBER 1
THEILGAARD-MÖNCH et al
interfere with its functional ability to bind Hb. However, it cannot
be ruled out that neutrophil- and plasma-derived Hp differ with
respect to anti-inflammatory activities. Hence, future studies are
needed to delineate the functional properties of the highly glycosylated Hp contained in specific granules of neutrophils.
The promoter region of the Hp gene harbors functionally
well-characterized APR-related binding sites, namely 2 C/EBP
sites and a binding site for the signal transducer and activator of
transcription 3 (STAT3).55,56 The proinflammatory cytokine IL-6
and glucocorticoids are principal regulators of Hp expression in
the human liver.57,58 IL-6 mediates the phosphorylation of
STAT3 in hepatocytes and enhances Hp expression through
binding of STAT3 and C/EBP to the STAT and C/EBP binding
sites, respectively.49,58
The hematopoietic growth factor G-CSF is an important
regulator of granulocyte differentiation, underscored by the finding
that mice deficient in G-CSF and the G-CSF receptor develop
severe neutropenia.59,60 As IL-6 signaling mediates STAT3 phosphorylation in the liver, G-CSF induces STAT3 phosphorylation through
the G-CSF receptor signaling pathway in myeloid cells.61,62 G-CSF
signaling and resultant STAT3 phosphorylation have been shown to
induce expression of the specific granule protein NGAL and the
transcription factor C/EBP⑀ in myeloid 32Dcl3 cells.54,63,64 In the
present study, we showed that C/EBP⑀ induces Hp expression in
myeloid 32Dcl3 cells. Consistent with our previous observation
that C/EBP⑀ mRNA and protein are highly expressed in vivo in
MYs when Hp is synthesized, these findings indicate that C/EBP⑀
contributes to the regulation of Hp expression during granulocyte
differentiation.31,53 Because MYs also express STAT3 and the
G-CSF receptor,31,65 one might speculate that G-CSF signaling
mediates a cooperative regulation of Hp expression by C/EBP⑀ and
STAT3 during granulocyte differentiation.
At present Hp is thought of as a plasma protein primarily
synthesized by hepatocytes to mediate anti-inflammatory activities
and immediate clearance of Hb released into plasma by erythrocytes as a consequence of hemolysis. However, the inflammatory
mediator LPS has been shown to induce Hp in other cell types,
including adipocytes, alveolar cells, and epidermal cells, suggesting a function for Hp at extravascular sites.66 In line with these
observations, the present study demonstrates that Hp, like other
specific granule proteins, is exocytosed within minutes by neutrophils after stimulation by PMA and various inflammatory stimuli.
In agreement with our previous findings that the specific granule
proteins Lf and human cathelicidin antimicrobial peptide are
exocytosed by neutrophils in vivo after migration into skin lesions,
the data presented here indicate that neutrophils represent the
primary local source of Hp at sites of infection or injury.67,68
In conclusion, the present study supports a model in which
neutrophils promote anti-inflammatory activities and clearance of
Hb at sites of infection or injury through the release of Hp in order
to reduce tissue damage and bacterial growth.
Acknowledgments
We thank Charlotte Horn and Marianne Lodahl their expert
technical assistance. K.T.M. is the recipient of a scholarship from
Rigshospitalet and the Danish Medical Research Council.
References
1. Gabay C, Kushner I. Acute-phase proteins and
other systemic responses to inflammation. N Engl
J Med. 1999;340:448-454.
13. Alayash AI. Hemoglobin-based blood substitutes:
oxygen carriers, pressor agents, or oxidants? Nat
Biotechnol. 1999;17:545-549.
2. Baumann H, Gauldie J. The acute phase response. Immunol Today. 1994;15:74-80.
14. Everse J, Hsia N. The toxicities of native and
modified hemoglobins. Free Radic Biol Med.
1997;22:1075-1099.
3. Wang Y, Kinzie E, Berger FG, Lim SK, Baumann
H. Haptoglobin, an inflammation-inducible
plasma protein. Redox Rep. 2001;6:379-385.
4. Wicher KB, Fries E. Prohaptoglobin is proteolytically cleaved in the endoplasmic reticulum by the
complement C1r-like protein. Proc Natl Acad Sci
U S A. 2004;101:14390-14395.
5. Wejman JC, Hovsepian D, Wall JS, Hainfeld JF,
Greer J. Structure and assembly of haptoglobin
polymers by electron microscopy. J Mol Biol.
1984;174:343-368.
6. Wejman JC, Hovsepian D, Wall JS, Hainfeld JF,
Greer J. Structure of haptoglobin and the haptoglobin-hemoglobin complex by electron microscopy. J Mol Biol. 1984;174:319-341.
15. Lim SK, Kim H, Lim SK, et al. Increased susceptibility in Hp knockout mice during acute hemolysis. Blood. 1998;92:1870-1877.
16. Lim YK, Jenner A, Ali AB, et al. Haptoglobin reduces renal oxidative DNA and tissue damage
during phenylhydrazine-induced hemolysis. Kidney Int. 2000;58:1033-1044.
17. Oh SK, Pavlotsky N, Tauber AI. Specific binding
of haptoglobin to human neutrophils and its functional consequences. J Leukoc Biol. 1990;47:
142-148.
18. Baseler MW, Burrell R. Purification of haptoglobin
and its effects on lymphocyte and alveolar macrophage responses. Inflammation. 1983;7:387-400.
7. Okazaki T, Yanagisawa Y, Nagai T. Analysis of the
affinity of each haptoglobin polymer for hemoglobin by two-dimensional affinity electrophoresis.
Clin Chim Acta. 1997;258:137-144.
19. Arredouani MS, Kasran A, Vanoirbeek JA, et al.
Haptoglobin dampens endotoxin-induced inflammatory effects both in vitro and in vivo. Immunology. 2005;114:263-271.
8. Kristiansen M, Graversen JH, Jacobsen C, et al.
Identification of the haemoglobin scavenger receptor. Nature. 2001;409:198-201.
20. Arredouani M, Matthijs P, Van Hoeyveld E, et al.
Haptoglobin directly affects T cells and suppresses T helper cell type 2 cytokine release. Immunology. 2003;108:144-151.
9. Moestrup SK, Moller HJ. CD163: a regulated hemoglobin scavenger receptor with a role in the
anti-inflammatory response. Ann Med. 2004;36:
347-354.
10. Fagoonee S, Gburek J, Hirsch E, et al. Plasma
protein haptoglobin modulates renal iron loading.
Am J Pathol. 2005;166:973-983.
21. El Ghmati SM, Van Hoeyveld EM, Van Strijp JG,
Ceuppens JL, Stevens EA. Identification of haptoglobin as an alternative ligand for CD11b/CD18.
J Immunol. 1996;156:2542-2552.
11. Eaton JW, Brandt P, Mahoney JR, Lee JT Jr.
Haptoglobin: a natural bacteriostat. Science.
1982;215:691-693.
22. Hanasaki K, Powell LD, Varki A. Binding of human plasma sialoglycoproteins by the B cell-specific lectin CD22: selective recognition of immunoglobulin M and haptoglobin. J Biol Chem.
1995;270:7543-7550.
12. Schaible UE, Kaufmann SH. Iron and microbial
infection. Nat Rev Microbiol. 2004;2:946-953.
23. El Ghmati SM, Arredouani M, Van Hoeyveld EM,
Ceuppens JL, Stevens EA. Haptoglobin interacts
with the human mast cell line HMC-1 and inhibits
its spontaneous proliferation. Scand J Immunol.
2002;55:352-358.
24. Yerbury JJ, Rybchyn MS, Easterbrook-Smith SB,
Henriques C, Wilson MR. The acute phase protein haptoglobin is a mammalian extracellular
chaperone with an action similar to clusterin. Biochemistry. 2005;44:10914-10925.
25. Faurschou M, Borregaard N. Neutrophil granules
and secretory vesicles in inflammation. Microbes
Infect. 2003;5:1317-1327.
26. Theilgaard-Monch K, Porse BT, Borregaard N.
Systems biology of neutrophil differentiation and
immune response. Curr Opin Immunol. 2006;18:
54-60.
27. Borregaard N, Cowland JB. Granules of the human neutrophilic polymorphonuclear leukocyte.
Blood. 1997;89:3503-3521.
28. Theilgaard-Monch K, Knudsen S, Follin P, Borregaard N. The transcriptional activation program of
human neutrophils in skin lesions supports their
important role in wound healing. J Immunol.
2004;172:7684-7693.
29. Le Cabec V, Cowland JB, Calafat J, Borregaard
N. Targeting of proteins to granule subsets is determined by timing and not by sorting: the specific
granule protein NGAL is localized to azurophil
granules when expressed in HL-60 cells. Proc
Natl Acad Sci U S A. 1996;93:6454-6457.
30. Cowland JB, Borregaard N. The individual regulation of granule protein mRNA levels during neutrophil maturation explains the heterogeneity of
neutrophil granules. J Leukoc Biol. 1999;66:989995.
31. Theilgaard-Monch K, Jacobsen LC, Borup R, et
al. The transcriptional program of terminal granulocytic differentiation. Blood. 2005;105:17851796.
32. Wagner L, Gessl A, Parzer SB, et al. Haptoglobin
From www.bloodjournal.org by guest on September 30, 2016. For personal use only.
BLOOD, 1 JULY 2006 䡠 VOLUME 108, NUMBER 1
phenotyping by newly developed monoclonal antibodies: demonstration of haptoglobin uptake
into peripheral blood neutrophils and monocytes.
J Immunol. 1996;156:1989-1996.
33. Wynn RF, Sood M, Theilgaard-Monch K, et al.
Intractable diarrhoea of infancy caused by neutrophil specific granule deficiency and cured by stem
cell transplantation. Gut. 2006;55:292-293.
34. Theilgaard-Monch K, Cowland J, Borregaard N.
Profiling of gene expression in individual hematopoietic cells by global mRNA amplification and
slot blot analysis. J Immunol Methods. 2001;252:
175-189.
35. Borregaard N, Sehested M, Nielsen BS, Sengelov H, Kjeldsen L. Biosynthesis of granule proteins
in normal human bone marrow cells: gelatinase is
a marker of terminal neutrophil differentiation.
Blood. 1995;85:812-817.
36. Staudinger BJ, Oberdoerster MA, Lewis PJ,
Rosen H. mRNA expression profiles for Escherichia coli ingested by normal and phagocyte oxidase-deficient human neutrophils. J Clin Invest.
2002;110:1151-1163.
37. Udby L, Calafat J, Sorensen OE, Borregaard N,
Kjeldsen L. Identification of human cysteine-rich
secretory protein 3 (CRISP-3) as a matrix protein
in a subset of peroxidase-negative granules of
neutrophils and in the granules of eosinophils.
J Leukoc Biol. 2002;72:462-469.
38. Calafat J, Janssen H, Stahle-Backdahl M, et al.
Human monocytes and neutrophils store transforming growth factor-alpha in a subpopulation of
cytoplasmic granules. Blood. 1997;90:12551266.
39. Kjeldsen L, Sengelov H, Borregaard N. Subcellular fractionation of human neutrophils on Percoll
density gradients. J Immunol Methods. 1999;232:
131-143.
40. Madsen M, Moller HJ, Nielsen MJ, et al. Molecular characterization of the haptoglobin hemoglobin receptor CD163: ligand binding properties of
the scavenger receptor cysteine-rich domain region. J Biol Chem. 2004;279:51561-51567.
41. Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage
T4. Nature. 1970;227:680-685.
42. Valtieri M, Tweardy DJ, Caracciolo D, et al. Cytokine-dependent granulocytic differentiation: regulation of proliferative and differentiative responses
in a murine progenitor cell line. J Immunol. 1987;
138:3829-3835.
43. Littlewood TD, Hancock DC, Danielian PS,
Parker MG, Evan GI. A modified oestrogen receptor ligand-binding domain as an improved switch
for the regulation of heterologous proteins.
Nucleic Acids Res. 1995;23:1686-1690.
Hp IS A SPECIFIC GRANULE PROTEIN OF NEUTROPHILS
44. Rasmussen T, Poulsen TS, Honore L, Johnsen
HE. Quantitation of minimal residual disease in
multiple myeloma using an allele-specific realtime PCR assay. Exp Hematol. 2000;28:10391045.
57.
45. Sorensen O, Arnljots K, Cowland JB, Bainton DF,
Borregaard N. The human antibacterial cathelicidin, hCAP-18, is synthesized in myelocytes and
metamyelocytes and localized to specific granules in neutrophils. Blood. 1997;90:2796-2803.
58.
46. Han H, Stessin A, Roberts J, et al. Calcium-sensing soluble adenylyl cyclase mediates TNF signal
transduction in human neutrophils. J Exp Med.
2005;202:353-361.
59.
47. Gombart AF, Koeffler HP. Neutrophil specific
granule deficiency and mutations in the gene encoding transcription factor C/EBP(epsilon). Curr
Opin Hematol. 2002;9:36-42.
48. Poli V, Mancini FP, Cortese R. IL-6DBP, a nuclear
protein involved in interleukin-6 signal transduction, defines a new family of leucine zipper proteins related to C/EBP. Cell. 1990;63:643-653.
49. Poli V. The role of C/EBP isoforms in the control
of inflammatory and native immunity functions.
J Biol Chem. 1998;273:29279-29282.
50. Lekstrom-Himes JA. The role of C/EBP(epsilon)
in the terminal stages of granulocyte differentiation. Stem Cells. 2001;19:125-133.
51. Lekstrom-Himes JA, Dorman SE, Kopar P, Holland SM, Gallin JI. Neutrophil-specific granule
deficiency results from a novel mutation with loss
of function of the transcription factor CCAAT/enhancer binding protein epsilon. J Exp Med. 1999;
189:1847-1852.
52. Yamanaka R, Barlow C, Lekstrom-Himes J, et al.
Impaired granulopoiesis, myelodysplasia, and
early lethality in CCAAT/enhancer binding protein
epsilon-deficient mice. Proc Natl Acad Sci U S A.
1997;94:13187-13192.
53. Bjerregaard MD, Jurlander J, Klausen P, Borregaard N, Cowland JB. The in vivo profile of transcription factors during neutrophil differentiation
in human bone marrow. Blood. 2003;101:43224332.
54. Verbeek W, Lekstrom-Himes J, Park DJ, et al.
Myeloid transcription factor C/EBP⑀ is involved in
the positive regulation of lactoferrin gene expression in neutrophils. Blood. 1999;94:3141-3150.
55. Oliviero S, Cortese R. The human haptoglobin
gene promoter: interleukin-6-responsive elements interact with a DNA-binding protein induced by interleukin-6. EMBO J. 1989;8:11451151.
56. Kim H, Baumann H. The carboxyl-terminal region
of STAT3 controls gene induction by the mouse
60.
61.
62.
63.
64.
65.
66.
67.
68.
361
haptoglobin promoter. J Biol Chem. 1997;272:
14571-14579.
Baumann H, Morella KK, Jahreis GP, Marinkovic
S. Distinct regulation of the interleukin-1 and interleukin-6 response elements of the rat haptoglobin gene in rat and human hepatoma cells.
Mol Cell Biol. 1990;10:5967-5976.
Ruminy P, Gangneux C, Claeyssens S, et al.
Gene transcription in hepatocytes during the
acute phase of a systemic inflammation: from
transcription factors to target genes. Inflamm
Res. 2001;50:383-390.
Lieschke GJ, Grail D, Hodgson G, et al. Mice
lacking granulocyte colony-stimulating factor
have chronic neutropenia, granulocyte and macrophage progenitor cell deficiency, and impaired
neutrophil mobilization. Blood. 1994;84:17371746.
Liu F, Wu HY, Wesselschmidt R, Kornaga T, Link
DC. Impaired production and increased apoptosis
of neutrophils in granulocyte colony-stimulating
factor receptor-deficient mice. Immunity. 1996;5:
491-501.
Chakraborty A, White SM, Schaefer TS, et al.
Granulocyte colony-stimulating factor activation
of Stat3 alpha and Stat3 beta in immature normal
and leukemic human myeloid cells. Blood. 1996;
88:2442-2449.
Tian SS, Lamb P, Seidel HM, Stein RB, Rosen J.
Rapid activation of the STAT3 transcription factor
by granulocyte colony-stimulating factor. Blood.
1994;84:1760-1764.
Wang L, Arcasoy MO, Watowich SS, Forget BG.
Cytokine signals through STAT3 promote expression of granulocyte secondary granule proteins in
32D cells. Exp Hematol. 2005;33:308-317.
Nakajima H, Ihle JN. Granulocyte colony-stimulating factor regulates myeloid differentiation
through CCAAT/enhancer-binding protein epsilon. Blood. 2001;98:897-905.
Klausen P, Bjerregaard MD, Borregaard N, Cowland JB. End-stage differentiation of neutrophil
granulocytes in vivo is accompanied by up-regulation of p27kip1 and down-regulation of CDK2,
CDK4, and CDK6. J Leukoc Biol. 2004;75:569578.
D’Armiento J, Dalal SS, Chada K. Tissue, temporal and inducible expression pattern of haptoglobin in mice. Gene. 1997;195:19-27.
Sengelov H, Follin P, Kjeldsen L, et al. Mobilization of granules and secretory vesicles during in
vivo exudation of human neutrophils. J Immunol.
1995;154:4157-4165.
Sorensen OE, Follin P, Johnsen AH, et al. Human
cathelicidin, hCAP-18, is processed to the antimicrobial peptide LL-37 by extracellular cleavage
with proteinase 3. Blood. 2001;97:3951-3959.
From www.bloodjournal.org by guest on September 30, 2016. For personal use only.
2006 108: 353-361
doi:10.1182/blood-2005-09-3890 originally published online
March 16, 2006
Haptoglobin is synthesized during granulocyte differentiation, stored in
specific granules, and released by neutrophils in response to activation
Kim Theilgaard-Mönch, Lars C. Jacobsen, Marianne J. Nielsen, Thomas Rasmussen, Lene Udby,
Maged Gharib, Peter D. Arkwright, Adrian F. Gombart, Jero Calafat, Søren K. Moestrup, Bo T. Porse
and Niels Borregaard
Updated information and services can be found at:
http://www.bloodjournal.org/content/108/1/353.full.html
Articles on similar topics can be found in the following Blood collections
Phagocytes (969 articles)
Red Cells (1159 articles)
Information about reproducing this article in parts or in its entirety may be found online at:
http://www.bloodjournal.org/site/misc/rights.xhtml#repub_requests
Information about ordering reprints may be found online at:
http://www.bloodjournal.org/site/misc/rights.xhtml#reprints
Information about subscriptions and ASH membership may be found online at:
http://www.bloodjournal.org/site/subscriptions/index.xhtml
Blood (print ISSN 0006-4971, online ISSN 1528-0020), is published weekly by the American Society
of Hematology, 2021 L St, NW, Suite 900, Washington DC 20036.
Copyright 2011 by The American Society of Hematology; all rights reserved.