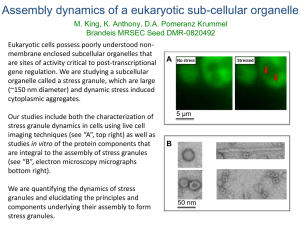
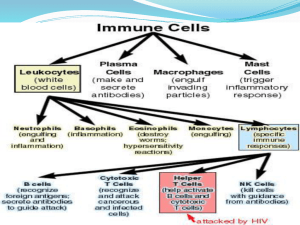
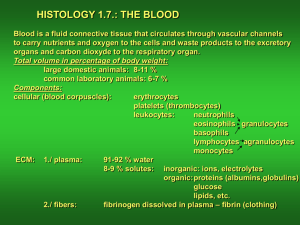
On how C. elegans spatially segregates Non-Membrane
advertisement

On how C. elegans spatially segregates Non-Membrane-Bound Compartments Christoph A. Weber Max Planck Institute for the Physics of Complex Systems, Division of Biological Physics P granules are cytoplasmic RNA/protein condensates known to determine the germ lineage in Caenorhabditis elegans. They resemble striking similarities with liquid droplets, such as dripping, shearing and wetting. Assuming that P granules are liquid-like we consider how they form in the crowded cytoplasm and how P granules are spatially segregated to one side of the cell. First P granule formation in-vivo and in-vitro are shown to share all hallmarks with a liquid-liquid phase-separation. Specifically, demixing in-vivo is determined by temperature and concentration and the droplet formation is reversible with respect to temperature quenches. The finding that P granules are formed by liquid-liquid demixing breaks the paradigmatic view that a molecular machinery is necessary to build up organelles through complex biological pathways. Liquid-liquid demixing could also serve as a mechanism for the assembly of non-membrane-bound compartments in other living organisms. Based on the insight that P granules can be regarded as liquid-like protein droplets we propose an extended Flory-Huggins model to describe the dynamics of P granule growth, interaction and spatial regulation. Specifically, guided by recent experimental observations we consider that PGL-3 competes with Mex-5 about mRNA and show that this mechanism is sufficient to explain that P granules only form at the side of the cell where the Mex-5 level is low. From the model we can compute quantitatively the gradient of supersaturation and study the ripening dynamics of the protein droplets. We find that ripening in a gradient is significantly accelerated making it to a rather efficient mechanism of concentrating material locally.