511
Cerebral Ischemic Attack Caused
by Postprandial Hypotension
Tomoyuki Kamata, MD; Takanori Yokota, MD; Tetsuo Furukawa, MD; Hiroshi Tsukagoshi, MD
Downloaded from http://stroke.ahajournals.org/ by guest on September 30, 2016
Background Food ingestion sometimes induces systemic
hypotension (postprandial hypotension). Although the possibility of stroke occurring postprandially has been suggested, no
cases have been reported until now.
Case Description A 78-year-old man experienced repeated
transient ischemic attacks after almost every ingestion of food
and showed orthostatic and postprandial hypotension. An
angiogram revealed occlusion of his left carotid artery and
stenosis of his right middle cerebral artery.
Conclusions Postprandial as well as orthostatic hypotension
can be a risk factor for stroke in patients with severe occlusive
cerebrovascular disease. (Stroke. 1994;25:511-513.)
Key Words • cerebral infarction • hemodynamics •
hypotension
F
often occurred after taking a hot bath, but he did not
complain of any symptoms of postural hypotension such
as fainting or lightheadedness. Magnetic resonance
imaging in May 1990 revealed multiple ischemic lesions
bilaterally in the deep cerebral white matter and normal
flow voids of the basilar and both internal carotid
arteries. At that time he was diagnosed as having
impaired glucose tolerance, but blood glycosylated hemoglobin content was normal. Because his blood pressure often was very high, the dose of his antihypertensive drug was increased. Thereafter, his attacks
consisting of dysarthria, right hemiparesis, and drowsiness occurred more frequently, almost after every meal,
and the duration also became longer, lasting more than
30 or 40 minutes. In August 1990 he again experienced
the attack after a meal, and since then his right hemiparesis has persisted. Moreover, he developed pneumonia. He was admitted to the hospital on August 23,1990.
On admission the patient was somnolent and had
severe right hemiparesis and speech disturbance. His
blood pressure fell from 124/68 to 101/59 mm Hg on
postural change from recumbent to sitting. He then
slavered from his right oral angle, and his right nasolabial fold became shallower. He became somnolent and
seemed aphasic, but in a few minutes he recovered.
Magnetic resonance imaging showed multiple ischemic lesions in the bilateral coronal radiation, thalamus,
basal ganglia, left ventromedial pons, and left cerebellar
hemisphere with a hyperintense signal in the T2weighed image. Moreover, the previously seen flow void
of the left internal carotid artery had disappeared. An
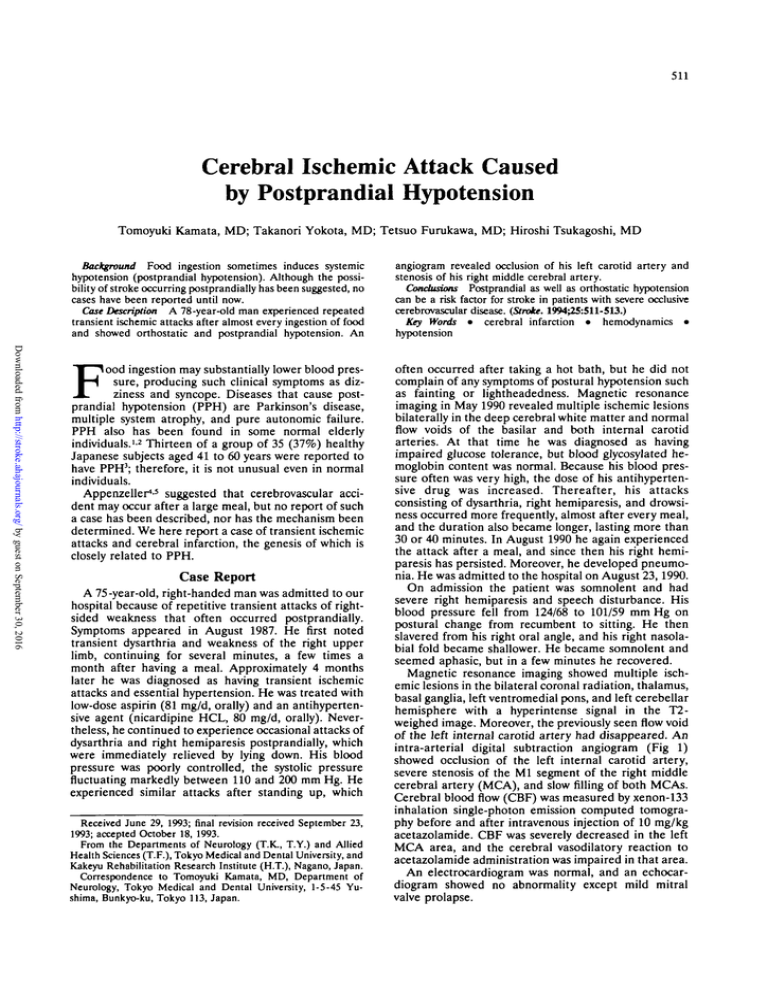
intra-arterial digital subtraction angiogram (Fig 1)
showed occlusion of the left internal carotid artery,
severe stenosis of the Ml segment of the right middle
cerebral artery (MCA), and slow filling of both MCAs.
Cerebral blood flow (CBF) was measured by xenon-133
inhalation single-photon emission computed tomography before and after intravenous injection of 10 mg/kg
acetazolamide. CBF was severely decreased in the left
MCA area, and the cerebral vasodilatory reaction to
acetazolamide administration was impaired in that area.
An electrocardiogram was normal, and an echocardiogram showed no abnormality except mild mitral
valve prolapse.
ood ingestion may substantially lower blood pressure, producing such clinical symptoms as dizziness and syncope. Diseases that cause postprandial hypotension (PPH) are Parkinson's disease,
multiple system atrophy, and pure autonomic failure.
PPH also has been found in some normal elderly
individuals.1'2 Thirteen of a group of 35 (37%) healthy
Japanese subjects aged 41 to 60 years were reported to
have PPH3; therefore, it is not unusual even in normal
individuals.
Appenzeller4-5 suggested that cerebrovascular accident may occur after a large meal, but no report of such
a case has been described, nor has the mechanism been
determined. We here report a case of transient ischemic
attacks and cerebral infarction, the genesis of which is
closely related to PPH.
Case Report
A 75-year-old, right-handed man was admitted to our
hospital because of repetitive transient attacks of rightsided weakness that often occurred postprandially.
Symptoms appeared in August 1987. He first noted
transient dysarthria and weakness of the right upper
limb, continuing for several minutes, a few times a
month after having a meal. Approximately 4 months
later he was diagnosed as having transient ischemic
attacks and essential hypertension. He was treated with
low-dose aspirin (81 mg/d, orally) and an antihypertensive agent (nicardipine HCL, 80 mg/d, orally). Nevertheless, he continued to experience occasional attacks of
dysarthria and right hemiparesis postprandially, which
were immediately relieved by lying down. His blood
pressure was poorly controlled, the systolic pressure
fluctuating markedly between 110 and 200 mm Hg. He
experienced similar attacks after standing up, which
Received June 29, 1993; final revision received September 23,
1993; accepted October 18, 1993.
From the Departments of Neurology (T.K., T.Y.) and Allied
Health Sciences (T.F.), Tokyo Medical and Dental University, and
Kakeyu Rehabilitation Research Institute (H.T.), Nagano, Japan.
Correspondence to Tomoyuki Kamata, MD, Department of
Neurology, Tokyo Medical and Dental University, 1-5-45 Yushima, Bunkyo-ku, Tokyo 113, Japan.
512
Stroke
Vol 25, No 2 February 1994
F o 1 . Right and left carotid anglcgrams. Left carotid angiogram
(right panel) shows disappearance
of left internal carotid arterial flow
due to total occlusion at the bifurcated portion from the left common
carotid artery. The collateral flow for
the left middle cerebral artery via
the ipsilateral ophthalmic artery is
also shown (arrowheads). Right carotid angiogram (left panel) shows
severe stenosis of the M1 segment
of the right middle cerebral artery
(arrow).
Downloaded from http://stroke.ahajournals.org/ by guest on September 30, 2016
An electroencephalogram showed a diffuse alpha
pattern while he was awake and diffuse 6- to 7-Hz theta
waves during drowsiness after meals.
During and after food ingestion, blood pressure was
monitored at 2-minute intervals with an automatic
sphygmomanometer (Fig 2). The patient had been
sitting up for more than 20 minutes, and he began to
eat while in the sitting position. His blood pressure
began to decrease during eating and reached a minimum approximately 10 minutes after he finished the
meal. Soon he slavered from his right oral angle and
his right nasolabial fold became shallower; he became
somnolent and aphasic within several minutes. When
attack
100
(mtn)
Urn*
FIG 2. Graph illustrates monitoring of blood pressure at
2-minute intervals during 20 minutes of sitting, then during and
after eating, including 80 minutes of lying down. Systolic blood
pressure decreased during the meal and reached a minimum
approximately 10 minutes afterward. A few minutes later the
patient became somnolent and seemed aphasic, with worsening
of his right facial hemiparesis . Those symptoms were relieved
by lying down.
he lay down, his blood pressure rose and those symptoms were relieved.
Upright tilt stimulation5 with blood sampling was
done to evaluate his postural hypotension. Blood pressure and heart rate were monitored continuously with
an automatic sphygmomanometer. An intravenous catheter was placed in his left femoral vein, and he was kept
recumbent for approximately 30 minutes before tilting.
Blood samples were collected just before tilting and at
5, 10, and 15 minutes while in the 60-degree upright
position. Blood pressure and heart rate while recumbent were 174/81 mm Hg and 66/min but changed to
126/65 mm Hg and 71/min, without any clinical symptoms, 5 minutes after the upright tilt. Plasma norepinephrine increased from 151 to 213 pg/mL. A Valsalva
maneuver for 10 seconds in the supine position failed to
produce a hypertensive overshoot. The cold pressor test
and the coefficient values of the RR intervals were
normal. To evaluate the hypotensive effect of glucose,
75 g of oral glucose was given in the 30-degree head-up
position. Blood samples were collected to measure
levels of glucose, insulin, and norepinephrine. The
patient's blood pressure before glucose loading (184/77
mm Hg) gradually decreased, reaching 124/61 mm Hg
105 minutes after loading. Serum glucose increased
from 90 to 184 mg/dL after the intake of glucose, and
plasma insulin increased slightly. There was no marked
change in the plasma norepinephrine.
After his fixed stroke, the patient occasionally experienced postprandial symptoms such as deterioration of
consciousness, right facial paresis, and aphasia. On the
basis of the report of Onrot et al,7 he was treated for
PPH with 250 mg caffeine orally per day, but with no
relief of his PPH or symptoms. Simply lying down on the
bed immediately after food intake had a more beneficial
effect on his postprandial symptoms.
Discussion
This case was characterized by repetitive, stereotyped
transient ischemic attacks after food ingestion. The
Kamata et al
Downloaded from http://stroke.ahajournals.org/ by guest on September 30, 2016
patient had left carotid occlusion and severe right MCA
stenosis. Moreover, he experienced orthostatic hypotension, and examinations of autonomic function revealed
impairment of the baroreflex. Impaired glucose tolerance might have been responsible in part for his PPH
and orthostatic hypotension, which an antihypertensive
agent could have exacerbated. However, we could not
be certain of the cause of his PPH and orthostatic
hypotension.
Stenosis or occlusion of one or more large cerebral
arteries might induce "states of cerebral vascular insufficiency" with recurrent symptoms reflecting hemodynamic crises.8 The role of systemic hypotension in
transient ischemic attack or stroke, however, is still a
matter of controversy. The recent work of Dobkin9
suggests that orthostatic hypotension can be a risk
factor for transient ischemic attacks if patients have
severe occlusive cerebrovascular disease. Orthostatic
hypotension in such patients is caused mainly by diabetes mellitus or the administration of antihypertensive
agents. Ordinarily, meals are taken in a sitting position.
In patients such as ours, who have both postural hypotension and PPH, the act of eating may result in severe
systemic hypotension.
In normal subjects the autoregulation system works
properly against hypotension so that CBF is maintained.
In humans1011 and experimental animals12 cerebral ischemia is known to impair autoregulation. These findings
suggest that in patients with severe occlusive cerebrovascular disease autoregulation cannot function fully
against the cerebral ischemia induced by a hypotensive
episode such as PPH or orthostatic hypotension. In
addition, chronic hypertension causes adaptive cerebral
vascular changes that lead to a shift of the lower limit of
autoregulation toward high pressure in young and middle-aged patients13 and in experimental animals.14'15
This functional adaptation is considered to be caused by
structural hypertensive vascular change,16 which in elderly hypertensive patients might become irreversible.17
Our patient's hypertension and cerebral ischemia may
have impaired his autoregulation.
In our patient's case it is clear that his stroke was
closely related to PPH. PPH therefore must be taken
into account in the management of patients with severe
occlusive cerebrovascular disease.
Acknowledgments
We are grateful to Kazuko Mitani, MD, and Hiroyuki Ohki,
MD, for their expert clinical advice.
Stroke Due to Postprandial Hypotension
513
References
1. Lipsitz LA Nyquist RP, Wei JY, Rowe JW. Postprandial reduction
in blood pressure in the elderly. N Engl J Med. 1983;309:81-83.
2. Lipsitz LA, Fullerton KJ. Postprandial blood pressure reduction in
healthy elderly. J Am Gcriatr Soc. 1986;34:267-270.
3. Otsuka K, Watanabe H. Postprandial hypotension observed by
ambulatory BP monitoring and BP reduction after oral glucose
loading [in Japanese with English abstract]. Jiritsushinkei 1990;27:
310-318.
4. Appenzeller O, Goss JE. Glucose and baroreceptor function:
effects of oral administration of glucose on baroreceptor function
in cerebrovascular disease and in other disorders with baroreceptor reflex block. Arch NeuroL 1970;23:137-146.
5. Appenzeller O. Postprandial hypotension. In: Appenzeller O, ed.
The Autonomic Nervous System. 4th ed. Amsterdam, the Netherlands: Elsevier Science Publishing Co, Inc; 1990:599-602.
6. Kaufmann H, Oribe E, Pierotti AR, Roberts JL, Yahr MD. Atrial
natriuretic factor in human autonomic failure. Neurology. 1990;40:
1115-1119.
7. Onrot J, Goldberg MR, Biaggioni I, Hollister AS, Kincaid D,
Robertson D. Hemodynamic and humoral effects of caffeine in
autonomic failure: therapeutic implications for postprandial hypotension. N Engl J Med. 1985;313:549-554.
8. Denny-Brown D. Recurrent cerebrovascular episodes. Arch
Neural 1960^:194-210.
9. Dobkin BH. Orthostatic hypotension as a risk factor for symptomatic occlusive cerebrovascular disease. Neurology. 1989;39:
30-34.
10. Fieschi C, Agnoli A, Battistini N, Bozzao L, Prencipe M.
Derangement of regional cerebral blood flow and of its regulatory
mechanisms in acute cerebrovascular lesions. Neurology. 1968; 18:
1166-1179.
11. Meyer JS, Shimazu K, Fukuuchi Y, Ohuchi T, Okamoto S, Koto A,
Ericsson AD. Impaired neurogenic cerebrovascular control and
dysautoregulation after stroke. Stroke, 1973;4:169-186.
12. Dirnagl U, Pulsinelli W. Autoregulation of cerebral blood flow in
experimental focal brain ischemia. J Cereb Blood Flow Metab. 1990;
10:327-336.
13. Strandgaard S. Autoregulation of cerebral blood flow in hypertensive patients: the modifying influence of prolonged antihypertensive treatment on the tolerance to acute, drug-induced hypotension. Circulation. 1976^3:720-727.
14. Jones JV, Fitch W, MacKenzie ET, Strandgaard S, Harper AM.
Lower limit of cerebral blood flow autoregulation in experimental
renovascular hypertension in the baboon. Ore Res. 1976;39:
555-557.
15. Barry DI, Strandgaard S, Graham DI, Braendstrup O, Svendsen
UG, Vorstrup S, Hemmingsen R, Bolwig TG. Cerebral blood flow
in rats with renal and spontaneous hypertension: resetting of the
lower limit of autoregulation. / Cereb Blood Flow Metab. 1982;2:
347-353.
16. Folkow B. The haemodynamic consequences of adaptive structural
changes of the resistance vessels in hypertension. Clin Set 1971;
41:1-12.
17. Strandgaard S. Cerebral blood flow in the elderly: impact of hypertension and antihypertensive treatment. Cardiovasc Drugs Ther.
1990;4:1217-1222.
Cerebral ischemic attack caused by postprandial hypotension.
T Kamata, T Yokota, T Furukawa and H Tsukagoshi
Stroke. 1994;25:511-513
doi: 10.1161/01.STR.25.2.511
Downloaded from http://stroke.ahajournals.org/ by guest on September 30, 2016
Stroke is published by the American Heart Association, 7272 Greenville Avenue, Dallas, TX 75231
Copyright © 1994 American Heart Association, Inc. All rights reserved.
Print ISSN: 0039-2499. Online ISSN: 1524-4628
The online version of this article, along with updated information and services, is located on the
World Wide Web at:
http://stroke.ahajournals.org/content/25/2/511
Permissions: Requests for permissions to reproduce figures, tables, or portions of articles originally published in
Stroke can be obtained via RightsLink, a service of the Copyright Clearance Center, not the Editorial Office.
Once the online version of the published article for which permission is being requested is located, click Request
Permissions in the middle column of the Web page under Services. Further information about this process is
available in the Permissions and Rights Question and Answer document.
Reprints: Information about reprints can be found online at:
http://www.lww.com/reprints
Subscriptions: Information about subscribing to Stroke is online at:
http://stroke.ahajournals.org//subscriptions/








