A Liquid Prism for Refractive Index Studies Michael D. Edmiston
advertisement

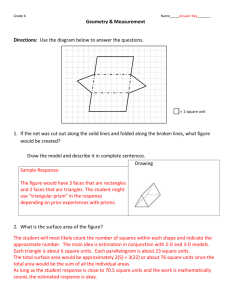
A Liquid Prism for Refractive Index Studies Michael D. Edmiston Department of Chemistry and Physics, Bluffton College, Bluffton, OH 45817 Helium-neon lasers are common in science labs; laser pointers are ubiquitous. Frequently teachers use these to demonstrate light refraction by a liquid.(1,2) Methods involve holding the liquid in a beaker, an Erlenmeyer flask, an aquarium, or various other containers. The easiest way to perform reproducible refraction experiments with liquids is to hold the liquid in a hollow, glass, equilateral, triangular prism. Constructed of flat glass plates and used properly, the glass contributes nothing to the overall refraction of the system.(1) The measured refraction is caused only by the wedge-shaped liquid; we essentially have a “liquid prism.” This paper describes an easy way to fabricate hollow glass prisms, the proper way to use these prisms, and an interesting demonstration using a laser pointer and hollow prism to measure the sugar content of soft drinks. We make hollow prisms by cutting and gluing standard 1"×3" microscope slides. The goal is an equilateral prism capable of holding liquid. For highly accurate refractive index measurements the prism angle must be known and the faces should be perfectly vertical. The prism angle can be measured with a spectrometer (1) and vertical faces can be achieved with careful glass cutting and careful alignment during gluing. For most experiments and for approximate refractive index measurements exactly 60° angles and exactly vertical faces are not necessary Place a piece of black electrical tape on a microscope slide as shown. Do not stretch it. The tape hangs over the edge. Score the opposite side with a diamond scribe (if available) or a glass cutter. Make the scribe lines one-inch apart and perpendicular to the long edge. Bend the glass toward the taped side just enough to break the glass at the scribe lines. (Break one at a time.) Bend the glass away from the tape, allowing the tape to stretch. Continue bending until the triangle closes. Set the prism on a flat surface to align the bottom edges. Use the overhanging tape to secure the prism in this configuration. It may be worthwhile to adjust the edges as necessary so they align correctly. We would like the inside edges in contact over the vertical length of each corner. Apply epoxy cement to the insides of the corners using a toothpick. Keep cement right at the corner and away from the faces. Then put epoxy along the bottom edge. Set the prism onto another slide (half a slide works well) allowing the epoxy to seal to this base. If desired, epoxy can be added to the outsides of the corners to make the prism stronger. Do not glue only the outsides because the prism will be weaker and can trap liquids in any voids at the seam. Hot-melt glue is a poor substitute for epoxy because it does not stick well to glass and often leaks or breaks. Silicone adhesives work well but have a fairly long cure time compared to 5-minute or even 30-minute epoxy. To use a prism with a laser, position the laser so the beam travels across the room and makes a spot on the wall. We locate the laser on the audience right 10-15 feet from the marker board on the front wall. The laser shines onto the audience left wall. When a prism is placed in front of the laser with the prism corner facing the audience, the refracted beam makes a spot on the front marker board. With this orientation higher refraction moves the spot toward audience right which seems appropriate. Marker Board Wall Refracted Path non-Refracted Path Hollow Prism Laser We do not want the laser to move during our experiments. We place a piece of masking tape on the wall where the non-refracted spot hits. This allows us to check periodically to make sure the laser has not moved. We use a wooden jig to hold the laser, and the jig also has a prism support a few inches from the laser and at the right height. The laser is fastened to the jig but the prism just sits on the support so it can be twisted around its vertical axis. The hollow prism does not yield a net refraction of light. Convince yourself by placing the empty prism in the laser path and noting the laser spot does not move. Now place water in the prism and observe a net refraction clearly caused by the wedge of water. For reproducible experiments and to determine the refractive index it is important to understand there is a minimum refraction angle for light passing through a wedge-shaped medium. Once the water-filled prism is in the laser path, rotate the prism back and forth around the vertical axis and note the refracted laser spot moves back and forth on the marker board. However, there is a minimum deviation of the beam. The minimum deviation occurs when the prism is oriented so the light passes symmetrically through the prism (the entrance angle and exit angle are the same and the path within the prism is perpendicular to the prism angle bisector). It is under these θ + θ MD sin P conditions the glass contributes absolutely nothing to the overall 2 refraction, and there is a simple formula relating the refractive index of the n= liquid to the prism angle (!P) and the minimum deviation angle (!MD). For θ sin P an equilateral prism !P is 60° and the minimum deviation angle can be 2 found with a protractor or by measuring distances and using the law of cosines. It is fun and worthwhile to have students measure the refractive index of water or other liquids and compare their results to those tabulated in handbooks. Tabulated values are for a wavelength of 589 nm rather than the 640-690 nm light from common lasers, but most liquids have low dispersion and the laser-measured values will be close to the published values. Some interesting experiments do not involve measurement of any angles or distances. These involve comparing the positions of the refracted laser spot as the liquid in the prism changes. Mark the un-refracted spot on the wall then mark the minimum refraction spots as some property of the liquid changes. For example if you try cold water and hot water the cold water shows more refraction. You can make a thermometer this way (but hot and cold liquids in the prism change temperature fairly quickly). This is a density effect. Actually it is an electron density effect so there is not a one-to-one correspondence between mass density and refractive index. For example, toluene has less mass density than water but has higher refractive index. However, if we study one substance, an increase in its mass density typically increases its refractive index, and lowering temperature is one way to effect this change. Another way to increase density is to dissolve some more-dense material in the liquid being studied. We can dissolve salt or sugar in water and observe a refractive index increase. If we prepare sugar-water solutions of known concentration, and mark their minimum refraction on the marker board, we can place unknown sugar-water solutions in the prism and estimate the sugar content from the position of the spot with respect to our standards. We like to measure the sugar content in soft drinks. We use common units by making sugar standards that are ½ level teaspoon of sugar per ounce of water, 1 teaspoon per ounce, and 1½ teaspoons per ounce. Most soft drinks fall between the ½ and 1 marks and interpolate to about e teaspoon sugar per ounce of water. This means a 12-ounce can contains about 7½ level teaspoons of sugar. This is reasonable agreement with the label on the can which reports the sugar content as grams of carbohydrates. This demonstration works with cola drinks because the coloring does not absorb much of the laser light. The carbonation can be a problem because the bubbles scatter the light. Sometimes we have to use a spatula to scrape bubbles from the walls of the prism so we can get a clean path through the prism. The demonstration also works with fruit juices including grape juice and orange juice if the room can be darkened. Just be sure the standard solutions and soft drinks are the same temperature, make sure the laser does not move, and always remember to adjust the prism to the minimum refraction. 1. Edmiston, M. D., Phy. Teach. 1986, 24, 160-163). 2. Hughes, Elvin; Jelks,Vaughn J. Chem. Educ. 1988, 65, 1007.