Comparison of Shortwave Diathermy and Microwave Diathermy
advertisement
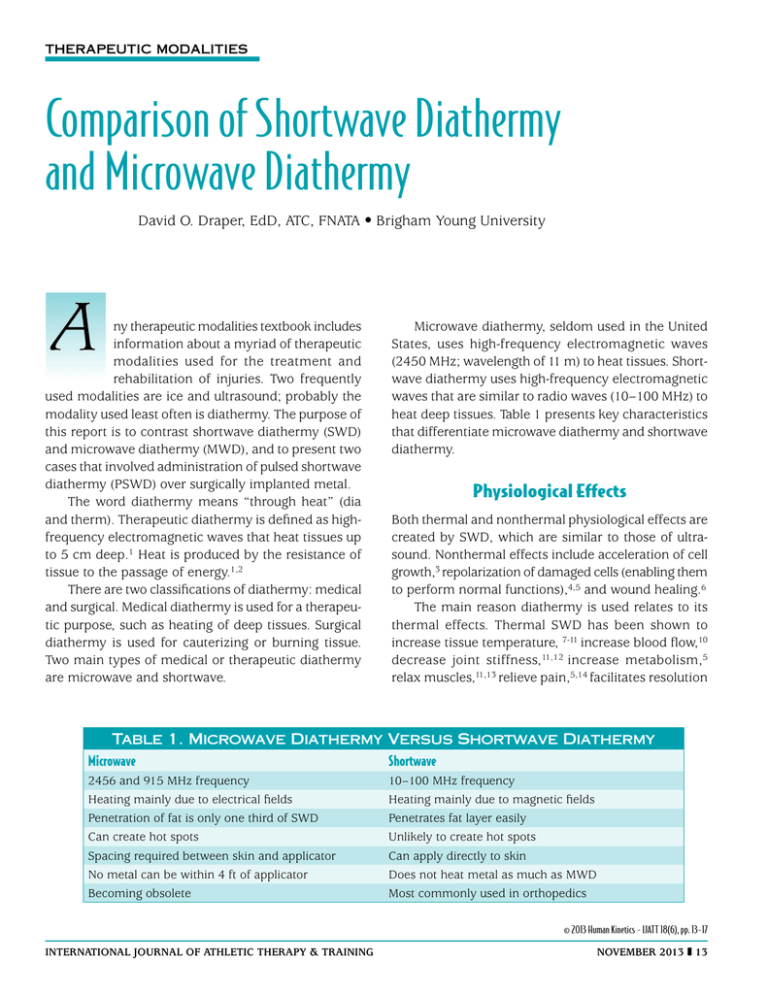
Therapeutic Modalities Comparison of Shortwave Diathermy and Microwave Diathermy David O. Draper, EdD, ATC, FNATA • Brigham Young University A ny therapeutic modalities textbook includes information about a myriad of therapeutic modalities used for the treatment and rehabilitation of injuries. Two frequently used modalities are ice and ultrasound; probably the modality used least often is diathermy. The purpose of this report is to contrast shortwave diathermy (SWD) and microwave diathermy (MWD), and to present two cases that involved administration of pulsed shortwave diathermy (PSWD) over surgically implanted metal. The word diathermy means “through heat” (dia and therm). Therapeutic diathermy is defined as highfrequency electromagnetic waves that heat tissues up to 5 cm deep.1 Heat is produced by the resistance of tissue to the passage of energy.1,2 There are two classifications of diathermy: medical and surgical. Medical diathermy is used for a therapeutic purpose, such as heating of deep tissues. Surgical diathermy is used for cauterizing or burning tissue. Two main types of medical or therapeutic diathermy are microwave and shortwave. Microwave diathermy, seldom used in the United States, uses high-frequency electromagnetic waves (2450 MHz; wavelength of 11 m) to heat tissues. Shortwave diathermy uses high-frequency electromagnetic waves that are similar to radio waves (10–100 MHz) to heat deep tissues. Table 1 presents key characteristics that differentiate microwave diathermy and shortwave diathermy. Physiological Effects Both thermal and nonthermal physiological effects are created by SWD, which are similar to those of ultrasound. Nonthermal effects include acceleration of cell growth,3 repolarization of damaged cells (enabling them to perform normal functions),4,5 and wound healing.6 The main reason diathermy is used relates to its thermal effects. Thermal SWD has been shown to increase tissue temperature, 7-11 increase blood flow,10 decrease joint stiffness,11,12 increase metabolism,5 relax muscles,11,13 relieve pain,5,14 facilitates resolution Table 1. Microwave Diathermy Versus Shortwave Diathermy Microwave Shortwave 2456 and 915 MHz frequency Heating mainly due to electrical fields Penetration of fat is only one third of SWD Can create hot spots Spacing required between skin and applicator No metal can be within 4 ft of applicator Becoming obsolete 10–100 MHz frequency Heating mainly due to magnetic fields Penetrates fat layer easily Unlikely to create hot spots Can apply directly to skin Does not heat metal as much as MWD Most commonly used in orthopedics © 2013 Human Kinetics - IJATT 18(6), pp. 13-17 international journal of Athletic Therapy & training november 2013 13 of hematomas,5 and increase extensibility of collagen fibers. 11,13 Table 2 presents recommended PSWD parameters for attainment of specific treatment goals. Many athletic trainers and therapists believe that ultrasound can heat deep tissues as well as diathermy. Although the magnitudes of tissue temperature elevation are similar, diathermy heats a much larger area than ultrasound.20 Ultrasound will heat up an area about as large as a catsup packet, whereas diathermy can heat up an area as large as a salad plate. Much of the widely-accepted misinformation about diathermy has failed to differentiate SWD from MWD. The contraindications of MWD have often been attributed to both types of treatment. This misinformation led to the widespread beliefs that no type of diathermy should be administered in the same room with a pregnant therapist,26 and that it should not be used over surgically implanted metal.2 These are both contraindications for MWD, but they are only precautions for SWD.5 In the text Therapeutic Modalities: The Art and Science,5 the Megapulse II PSWD device is described as safe for use over metal implants, based on years of using this device on patients with surgically implanted metal rods, plates, and screws.5 However, no form of diathermy treatment should be administered over looped wires, which can act as an antenna and overheat surrounding tissues. Also, SWD should be pulsed (PSWD), not continuous.5 Why Diathermy Is Seldom Used Diathermy has fallen out of favor among athletic trainers and therapists in many parts of the world, with the exceptions of the United Kingdom and Japan. Surveys of physical therapists in Canada15 and Australia16 have documented that diathermy was used on a daily basis by 0.6% and 8% respectively, whereas ultrasound was used daily by 93.5%.15,16 Diathermy is seldom used in the United States, which is probably due to its expense, outdated research, and misinformation. The price of a diathermy device can range from USD $6,000 to $25,000. Because many insurance companies do not provide reimbursement for diathermy treatments, insufficient revenue is likely to be generated to cover the cost of acquiring the equipment. Prior to the last 15 years,7-11,17-25 little research evidence of therapeutic benefit from diathermy existed. Many improvements in the function of the devices started taking place in the 1990s. One improvement was proper shielding from electromagnetic waves, which improved safety for both the patient and the clinician. There also can be a significant difference in heating effect between various SWD devices. A recent study24 compared the heating effect of the ReBound SWD device (ReGear Life Sciences, Inc., Pittsburg, PA) and the Megapulse II SWD device (Accelerated Care Plus, Reno, NV). Temperature probes were inserted 3 cm into the triceps surae of 12 human subjects. Treatments were administered for 30 minutes. The Megapulse II SWD device increased the muscle temperature 4.32°C, whereas the ReBound SWD device increased the temperature only 2.3°C.24 Case Report 1 A 38-year-old construction worker fell two stories onto a concrete floor. The impact collapsed a lung and caused a humerus fracture. The fractured humerus was surgically repaired with a metal rod. At approximately 6 months postinjury, two thermocouples were inserted into his upper arm to measure the tissue heating effect of PSWD. One thermocouple was inserted until it came into contact with the metal at a depth of 2.6 cm. The other thermocouple was inserted into muscle tissue at the same depth (Figure 1). The Megapulse II drum Table 2. Shortwave Diathermy Parameters for Specific Treatment Goals Dose Indications Pulse width Pulse rate Average watts n/a Acute trauma, edema reduction, cell repolarization, and repair Subacute inflammation Pain reduction, muscle spasm, chronic inflammation Stretch collagen-rich tissues 65 μs 100–200 pps n/a 100–200 μs 200–400 μs 400–800 pps 400–800 pps 12 24 400 μs 800 pps 48 1°C 2°C 4°C 14 november 2013 international journal of Athletic Therapy & training (Accelerated Care Plus, Reno, NV) was applied to the skin over the thermocouples. A 20-minute diathermy treatment was administered at 27.12 MHz, 800 μsec, 400 Hz, and 100 Watts average power (Figure 2). Previous research has documented that this technique will raise the temperature in the deep tissues over 4°C.1 The temperature was recorded every 10 minutes. At the end of the 20-minute treatment, the temperature had increased 4.5°C on the metal rod and the surrounding muscle. Case Report 2 pronation and supination. Four months earlier, she had dislocated the elbow and fractured her distal humerus when she fell during a gymnastics event (Figure 3). The humerus fracture was surgically repaired with titanium pins and screws. After several weeks of immobilization, she received extensive physical therapy treatments (ultrasound and passive stretching). Her full range of elbow flexion was restored, but elbow extension, forearm pronation, and forearm supination were restricted. The treatment regimen consisted of 20 minutes of PSWD at an average of 48 Watts (Figure 4), which was followed by 8–10 minutes of joint mobilization A female collegiate gymnast presented an elbow that she could not extend beyond the last 30° of the normal arc of motion. She also lacked full range of motion in Figure 1 Patient 1 with a metal rod in his humerus. Note the two temperature probes in his tissue. Figure 3 Radiograph of patient 2. Figure 2 Patient 1 receiving PSWD treatment. Figure 4 Beginning ROM for patient 2. international journal of Athletic Therapy & training november 2013 15 (traction, anterior glides of the distal humerus on the ulna, and radial head glides). After seven treatments, she had attained full range of motion in extension, pronation, and supination (Figure 5). implant should be viewed as a treatment precaution. However, any type of diathermy treatment is always contraindicated over metal implants that have circular wire components or elements that form loops, which may result in excessive heating of the metal.2,5,26 References Figure 5 Full ROM was reached with a regimen of PSWD and joint mobilizations. Discussion One of the most promising applications of PWSD is facilitation of stretching and joint mobilization for restoration of joint range of motion.11,13,17-19,21,23 A heat and stretch regimen using PSWD can increase the flexibility of subjects with tight muscles,17 and improve the joint mobility of patients with limited ankle dorsiflexion.22 These effects likely result from reduction of muscle guarding and pain, decreased tissue stiffness,22 and increased extensibility of collagen fibers.5-7,11-13 Many clinicians believe that the administration of PSWD over surgically implanted metal screws, rods, or pins is contraindicated.2,5,18,19,22 The close proximity of a radio frequency field to a metal implant is widely believed to cause a greater temperature increase in the adjacent tissue than in tissue that does not contain a metal implant.2,5,18,19,22 This concern should be viewed as more of a precaution than a contraindication, because therapeutic benefits have been documented when the power level was 100 Watts or less.5 Conclusion Pulsed shortwave diathermy (< 100 Watts) is a safe therapeutic procedure, and the presence of a metal 16 november 2013 1.Draper DO, Knight KL, Fujiwara T, Castel JC. Temperature change in human muscle during and after pulsed shortwave diathermy. J Orthop Sports Phys Ther. 1999;29(1):13-18. 2.Cameron MH. Physical Agents in Rehabilitation; From Research to Practice. 2nd ed. Philadelphia, PA: WB Saunders, 2003. 3.Canaday D, Lee R. Scientific basis for clinical application of electric fields in soft tissue repair. In: Brighton C, Pollack S, eds. Electromagnetics in Biology Medicine. San Francisco, CA: San Francisco Press, 1991. 4.Low JL. Dosage of some pulsed shortwave clinical trial. Physiotherapy.1995, 81(10):611-614. 5.Knight KL, Draper DO. Therapeutic Modalities: The Art and Science. 2nd ed. Baltimore, MD, Lippincott Williams & Wilkins; 2013:283-302. 6. Bricknell R, Watson T. The thermal effects of pulsed shortwave therapy. Br J Ther Rehabil. 1995; 2:430-434. 7. Draper DO. Interest in diathermy heats up again. Biomechanics. 2001, 8 (9):77-83. 8. Draper DO, Garrett C. Pulsed shortwave diathermy heats a considerable larger area than 1 MHz ultrasound treatments. J Athl Train. 1999, 34(2):21-23. 9.Draper DO, Abergel PA, Castel JC. Pulsed shortwave diathermy restricts swelling and bruising of liposuction patients. Am J Cosmet Surg. 2000;17(1):17-22. 10. Jan M, Lip P, Lin K. Change of arterial blood flow and skin temperature after direct and indirect shortwave heating on knee. Formosan J Phys Ther. 1993;18:64-71. 11.Brucker JB, Knight K, Rubley M, Draper DO. Effect of an 18-day stretching regimen, with or without PSWD on ankle dorsiflexion and 3 weeks. J Athl Train. 2005;40(4):189-193. 12. Kaltenborn FM. Manual Mobilization of the Joints. Minneapolis, MN: OTPT, 2002. 13.Draper DO. Induction cable diathermy and joint mobilization restore range of motion in a post-operative ACL patient. Athl Ther Today. 2010;15(1):36-38. 14.Svarcova J, Trnavsky, Avarova J. The influence of ultrasound galvanic currents and shortwave diathermy on pain intensity in patients with osteoarthritis. Scand J Rheumatol. 1987;67:83-85. 15.Lindsay DM, Dearness J, McGinley CC. Electrotherapy usage trends in private physiotherapy practice in Alberta. Physiother Can. 1995;47(1):30-34. 16.Lindsay DM, Dearness J, Richardson C, Chapman A, Cuskelly G. A survey of electromodality usage in private physiotherapy practices. Aust J Physiother. 1990;36(4):249-256. 17.Draper DO, Castro J, Feland JB, Schulthies SS, Eggett D. Shortwave diathermy and prolonged stretching increase flexibility more than stretching alone. J Orthop Sports Phys Ther. 2003;34(1):13-20. 18. Draper DO, Castel JC, Castel D. Low-watt pulsed shortwave diathermy and metal plate fixation of the elbow. Athl Ther Today. 2004;9(5):27-31. 19. Draper DO. Shortwave diathermy in the athletic training room. Paper presented at: Annual Symposium of the NATA; Los Angeles , CA, June 2001. 20.Garrett CL, Draper DO, Knight KL. Heat distribution in the lower leg from pulsed shortwave diathermy and ultrasound treatments. J Athl Train. 2000;35(1):13-22. international journal of Athletic Therapy & training 21.Peres S, Draper DO, Knight KL, Ricard MD, Durrant E. Pulsed shortwave diathermy used prior to stretch increases flexibility more than stretch alone. J Athl Train. 2000;37(1):43-50. 22.Seiger C, Draper DO. Use of pulsed shortwave diathermy and joint mobilization to increase range of motion in the presence of surgical implanted metal: A case series. J Orthop Sports Phys Ther. 2006;36(9):669-677. 23. Draper DO. Shortwave diathermy and joint mobilizations for postsurgical restoration of knee motion. Athl Ther Today. 2010;15(1):38-40. 24.Draper DO, Hawkes A, Johnson AW, Diede M, Rigby J. Muscle heating with the Megapulse II shortwave diathermy and ReBound diathermy. J Athl Train. 2013;48(4):477-482. international journal of Athletic Therapy & training 25. Hawkes A, Draper DO, Johnson AW, Diede M, Rigby J. Heating capacity of ReBound shortwave diathermy and moist hot packs at superficial depths. J Athl Train. 2013;48(4):471-476. 26.Hellstrom RO, Stewart WF. Miscarriages among female physical therapists who report using radio- and microwave-frequency electromagnetic fields. Am J Epidemial. 1993;138(10):775-785. David O. Draper is a professor in the Exercise Sciences division at Brigham Young University in Provo, UT. Lindsey Eberman, PhD, ATC, LAT, Indiana State University, is the report editor for this article. november 2013 17








