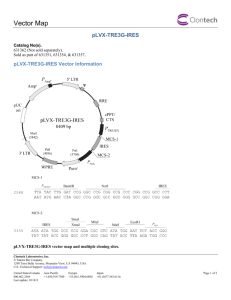
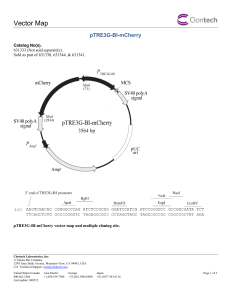
Tet-On® 3G Inducible Expression System
advertisement

Tet-On® 3G Inducible Expression System Mammalian Expression Systems Lowest background, highest sensitivity The Tet-On 3G Tetracycline Inducible Gene Expression Systems are the 3rd generation of the most powerful, versatile, and widely cited inducible mammalian expression systems available. They provide precisely regulated control of transgene expression that is reversible, quantitative, and reproducible. The 3G system offers a significant improvement over the exquisite Tet System technologies developed in the laboratories of Hermann Bujard, Manfred Gossen, and Wolfgang Hillen (1, 2) by combining a new promoter that shows significantly reduced background (3), and a new transactivator protein with increased sensitivity (4). How Do Tet-On 3G Inducible Systems Work? Target cells that express the Tet-On 3G transactivator protein and contain a gene of interest (GOI) under the control of a TRE3G promoter (PTRE3G) will express high levels of your GOI, but only when cultured in the presence of doxycycline (Dox), a tetracycline analog. When bound by Dox, the Tet-On 3G protein undergoes a conformational change that allows it to bind to tet operator (tetO) sequences located in PTRE3G (Figure 1). Unlike inferior TetR-based systems (never sold by Clontech), Tet-On technologies activate rather than repress transcription, a critical difference which results in far lower basal expression, higher maximal expression, a more rapid response time—and ultimately, a system proven to be the first choice for conditional expression in vivo. No Dox Tet-On 3G Transactivator PTRE3G Promoter Dox Doxycycline Transcription Figure 1. The Tet-On 3G systems allow inducible gene expression only in the presence of doxycycline. When Dox binds, the transactivator undergoes a conformational change allowing it to bind tet operator (tetO) repeats within the TREG Promoter (PTRE3G). The transactivator activates expression through transcription activation domain repeats. Clontech Laboratories, Inc. A Takara Bio Company www.clontech.com United States/Canada: +1.800.662.2566 • Asia Pacific: +1.650.919.7300 • Europe: +33.(0)1.3904.6880 • Japan: +81.(0)77.543.6116 For Research Use Only. Not for use in diagnostic or therapeutic procedures. Not for resale. Clontech, the Clontech logo, and all other trademarks are the property of Clontech unless noted otherwise. ©2010 Clontech Laboratories, Inc. AN0Z3745 IN Tet-On® 3G …continued Lowest-Ever Background, Highest Sensitivity The combination of the following two optimized elements makes Tet-On 3G better than previous generations of the Tet-On system (Figures 2 & 5, Table I): 1. PTRE3G promoter—mutations have reduced background expression from the inducible promoter by up to 20-fold (Figure 2). 2. Tet-On 3G transactivator protein—mutations have significantly increased its sensitivity to the inducer doxycycline (Dox), so that at very low Dox concentrations (between 5–10 ng/ml), expression can be 100–150 fold higher when using a Tet-On 3G system compared to Tet-On Advanced (Figure 3). When the two elements are combined, not only can you detect very high expression of your protein after exposure to just 10 ng/ml of Dox (Figure 4), but the background is so low that even in transient cotransfections we have seen up to a 27,000-fold difference in expression between the induced and uninduced state (MCF7 cells, data not shown). 160,000 80,000 Tet-On Advanced Tet-On 3G Reduced Background 600 0 Tet-On 3G 12,000 8,000 4,000 40,000 0 Transactivator Tet-On Advanced RLU RLU 120,000 16,000 System 800 0 50 ng/ml 0 500 0 5 10 50 Doxycycline concentration (ng/ml) 100 Figure 2. The PTRE3G promoter results in significantly reduced basal expression. HEK 293 cells were transiently cotransfected with both the response vectors (containing luciferase) and regulator vectors from each of the Tet-On 3G and Tet-On Advanced Inducible Expression Systems. The cells were cultured in the presence and absence of Dox, and after 24 hr, luciferase expression was measured. Although both systems provided strong expression in the presence of Dox, the Tet-On 3G System produces far lower background expression in the absence of Dox (inset). Figure 3. Tet-On 3G demonstrates higher sensitivity to doxycycline than Tet-On Advanced. Tet-On 3G and Tet-On Advanced genes were integrated at the same locus in a stable HLF33 cell line expressing luciferase from a TRE promoter. For each of these two double-stable cell lines, induced luciferase expression was measured in response to a range of doxycycline (Dox) concentrations. At 5–10 ng/ml Dox, induced expression was 100–150-fold higher for the Tet-On 3G cell line, and at 50 ng/ml, expression was 4.6 fold higher (data kindly provided by Professor W. Hillen and Dr. C. Berens, University of Erlangen). A B kD 0 10–1 Dox (ng/ml) 10 0 101 102 103 50– pTRE3G-Luc 160,000 120,000 Luciferase RLU 70– b-Actin 80,000 40,000 0 0 10–¹ 100 10¹ 10² 10³ ng/ml Figure 4. Tet-On 3G is highly sensitive to as little as 10 ng/ml of doxycycline. Following cotransient transfection of pCMV-Tet3G and pTRE3G-Luc in HeLa cells, increasing levels of Dox were added and expression of luciferase was measured using an anti-luciferase antibody (Panel A) and a luciferase assay (Panel B). Induced expression was very high even with Dox concentrations as low as 10 ng/ml, and was detectable even at 0.1 ng/ml Dox. Clontech Laboratories, Inc. • www.clontech.com 2 Tet-On® 3G …continued The Tet-On 3G System is a Powerful Combination of Two Elements Optimized by Mutation Tet-On 3G Transactivator Protein Tet-On 3G is a modified form of an improved Tet-On Advanced transactivator protein which has been evolved to display far higher sensitivity to doxycycline (4). The protein consists of a modified bacterial Tet repressor (TetR) fused to three minimal VP16 activation domains to create a transcriptional activator protein (transactivator). Five amino acid changes convert Clontech’s Tet-On Advanced transactivator to Tet-On 3G (for a comparison of the various Tet-On transactivator sequences, see www.clontech.com). Tet-On 3G generates very high maximal expression and responds to lower Dox concentrations than its predecessors. These Dox concentrations are far below cytotoxic levels for either cell culture or transgenic studies. The increased Dox sensitivity is particularly advantageous for in vivo studies in tissues where high Dox concentrations are difficult to attain (e.g., brain). Table I: Three Generations of Tet-On Inducible Expression Systems Generation Transactivator Protein Inducible Promoter Tet-On System Name 1st Tet-On PTRE2 Tet-On Advanced System 2nd Tet-On Advanced PTIGHT Tet-On 3G System 3rd Tet-On 3G PTRE3G HEK 293 Cells HeLa Cells 1,600 8,000 Tet-On Tet-On Advanced Fold induction Tet-On 3G 1,200 6,000 800 4,000 400 2,000 0 1st 2nd Generation 3rd 0 1st 2nd Generation 3rd Figure 5. Comparison of the three generations of Tet-On. Panel A shows the transactivator and inducible promoter combinations for each generation of the Tet-On system. Tet-On was launched by Clontech in 1996—at the time this was the premier inducible expression system, and its performance has only been surpassed by subsequent generations of the Tet-On system. Panel B shows co-transient transfection experiments with both vectors in HEK 293 and HeLa cells. Although Tet-On Advanced shows great improvement over the original Tet-On System when comparing the difference between the induced and the uninduced states (fold induction), Tet-On 3G shows even higher fold induction due to the significantly reduced basal expression provided by the PTRE3G promoter. Clontech Laboratories, Inc. • www.clontech.com 3 Tet-On® 3G …continued PTRE3G Inducible Promoter The inducible promoter PTRE3G provides for very low basal expression and high maximal expression after induction (3). It consists of 7 repeats of a 19 bp tet operator sequence located upstream of a minimal CMV promoter, which we refer to as the tetracycline response element (TRE). Although the sequences of tet operator repeats are identical in all Tet-On generations (Figure 6), the junction sequences of PTRE3G have been altered to an even spacing and the central portions are randomized. Additionally, elements from the minimal CMV promoter have been mutated to consensus. Because PTRE3G lacks binding sites for endogenous mammalian transcription factors and is inactive in the absence of a transactivator protein, basal expression from PTRE3G is the lowest of any TRE-containing promoter available so far. TRE Promoter Alignment tetO tetO T TTACCACTCCCTATCAGTGATAGAGAAAAGTGAAAGTCGAGTTTACCACTCCCTATCAGTGATAGAGAAAA-GTGAAAGTC PTRE2 (1) PTight (1) PTRE3G (1) T TT---ACTCCCTATCAGTGATAGAGAAC---GTATGAAGAGTTTA---CTCCCTATCAGTGATAGAGAACGTATG----CA PTRE2 (82) PTight G AGTTTACCACTCCCTATCAGTGATAGAGAAAAGTGAAAGTCGAGTTTACCACTCCCTATCAGTGATAGAGAAAAGTGAAAG (69) T TT---ACTCCCTATCAGTGATAGAGAAC---GTATGTCGAGTTTA---CTCCCTATCAGTGATAGAGAACG-ATG----TC tetO tetO G AGTTTAC---TCCCTATCAGTGATAGAGAAC---GTATGTCGAGTTTA---CTCCCTATCAGTGATAGAGAAC---GTATG PTRE3G (70) G A C T T T A C - - - T C C C T A T C A G T G A T A G A G A A C - - - G T A T A A G G A G T T T A - - - C T C C C T A T C A G T G A T A G A G A A C - - - G T A T G tetO tetO PTRE2 (164) T C G A G T T T A C C A C T C C C T A T C A G T G A T A G A G A A A A G T G A A A G T C G A G T T T A C C A C T C C C T A T C A G T G A T A G A G A A A A G T G A A PTight (139) T C G A G T T T A C - - - T C C C T A T C A G T G A T A G A G A A C - - - G T A T G T C G A G T T T A - - - - T C C C T A T C A G T G A T A G A G A A C - - - G T A PTRE3G (140) A C C A G T T T A C - - - T C C C T A T C A G T G A T A G A G A A C - - - G T A T C T A C A G T T T A - - - C T C C C T A T C A G T G A T A G A G A A C - - - G T A tetO Modified Minimal CMV Promoter PTRE2 (246) A G T C G A G T T T A C C A C T C C C T A T C A G T G A T A G A G A A A A G T G A A A G T C G A G C T C G G T A C C C G G G T C G A G G T A G G C G T G T A C G G T PTight (208) T G T C G A G T T T A C - - - T C C C T A T C A G T G A T A G A G A A - - C - - - - - - - - - - - - - - - G T A T - - - - G T C G A G G T A G G C G T G T A C G G T PTRE3G (210) T A T C C A G T T T A C - - - T C C C T A T C A G T G A T A G A G A A - - - - - - - - - - - - C - - - - - G T A T - - - - - A A G C T T T A G G C G T G T A C G G T Modified Minimal CMV Promoter (cont.) PTRE2 (328) G G G A G G C C T A T A T A A G C A G A G C T C G T T T A G T G A A C C G T C A G A T C G C C T G G A G A C G C C A T C C A C G C T G T T T T G A C C T C C A T A G PTight (266) G G G A G G C C T A T A T A A G C A G A G C T C G T T T A G T G A A C C G T C A G A T C G C C T G G A - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - PTRE3G (267) G G G C G - C C T A T A A A A G C A G A G C T C G T T T A G T G A A C C G T C A G A T C G C C T G G A G C A A T - - T C C A C A A C A C T T T T G T C T T A T A C C PTRE2 (410) A A G A C A C C G G G A C C G A T C C - - - - - - - - - - - PTight (317) - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - PTRE3G (346) A A C T T T C C G T A C C A C T T C C T A C C C T C G T A A A Figure 6. Sequence comparison of the three generations of TRE promoters sold by Clontech. Each promoter consists of 7 identical repeats of the tet operator sequence (green), and contains a minimal CMV promoter—although this sequence has been mutated to consensus in PTRE3G (3). Moreover, in PTRE3G the spacer sequences between the tetO repeats are evenly spaced and contain randomized central sequences. Combining these two components (the PTRE3G promoter and Tet-On 3G transactivator) results in the largest difference between the induced and uninduced states (fold induction) of any commercially available inducible expression system for mammalian cells (Figure 5). Clontech Laboratories, Inc. • www.clontech.com 4 Tet-On® 3G …continued Choice of Tet-On 3G Vector Formats Figure 7 shows the range of Tet-On 3G vector formats available. Each is sold as a complete system that includes: • a Tet-On transactivator vector (either pCMV-Tet3G or pEF1a-Tet3G) • a TRE response vector with a PTRE3G promoter and a multiple cloning site • both hygromycin and puromycin linear selection markers • Tet Approved FBS • Xfect™ transfection reagent Transactivator Vectors pCMV-Tet3G expresses the Tet-On 3G protein constitutively from a CMV promoter, and is included in all Tet-On 3G systems except the EF1 alpha version, in which an EF-1 alpha promoter expresses the transactivator. pCMV-Tet3G PCMV IE Tet-On 3G PSV40 Neor Tet-On 3G PSV40 Neor pEF1a-Tet3G PEF-1a Response Vectors All response vectors contain a PTRE3G promoter. pTRE3G is included in both the core system and the EF-1 alpha version. pTRE3G-IRES can inducibly coexpress any two genes of interest, and is included with the bicistronic Tet-On 3G system. Alternatively, you can monitor inducibility using red or green fluorescent proteins if you are using the mCherry or ZsGreen1 systems. PTRE3G MCS pTRE3G pA MCS PTRE3G MCS pTRE3G-IRES IRES pA IRES MCS PTRE3G mCherry pA IRES MCS pTRE3G-mCherry pA pTRE3G-ZsGreen1 PTRE3G ZsGreen1 Linear Selection Markers Selection for inducible clones can be performed using either hygromycin or puromycin linear selection markers. Both markers are included with every Tet-On 3G system. Linear Hygromycin Marker PSV40 Hygr pA Linear Puromycin Marker PSV40 Puror pA Figure 7. Vector formats for Tet-On 3G systems. For complete components lists, see www.clontech.com Clontech Laboratories, Inc. • www.clontech.com 5 Tet-On® 3G …continued Inducible Expression in Stem Cells and Hematopoietic Cells The Tet-On 3G Inducible Expression System (EF1a Version), our EF-1 alpha promoter version of the Tet-On 3G system, provides for consistent long-term expression of the Tet-On 3G transactivator, even in cell types known for their tendency to silence a CMV promoter over time, such as hematopoietic cells and stem cells (Figure 7). We tested the EF-1 alpha version in Jurkat cells, a cell line known to show reduced expression and clonal variation in expression from CMV- based vectors. When expressing the Tet-On 3G transactivator protein from the EF-1 alpha promoter, 83% of the Jurkat Tet-On 3G clones showed strong inducible expression and 33% demonstrated very high inducibility (greater than 2,000-fold). Such levels of control are not possible when using previous versions of the Tet-On system for this cell line (Figure 8). The EF-1 alpha promoter is a preferred promoter for gene expression in stem cells. We demonstrated that the Tet-On 3G Inducible Expression System (EF1a version) displays excellent performance in mouse embryonic stem cells (Figure 9). Jurkat Tet-On 3G Cells 200,000 – Dox + Dox 160,000 RLU 120,000 80,000 40,000 0 1 2 3 4 5 6 7 8 9 10 Clone no. 11 12 13 14 15 16 17 18 Figure 8. Tet-On 3G (EF1α Version) provides inducible expression in hematopoietic cells. Jurkat cells were transfected with pEF1α-Tet3G using Xfect transfection reagent, and stable clones were selected by limiting dilution. 18 stable clones were then tested for inducibility by transient transfection using pTRE3G-expressing luciferase. Six out of eighteen clones showed more than 2,000-fold induction via transient transfection. Mouse Embryonic Stem Cells Dox No Dox A B C D Figure 9. Tet-On 3G (EF1α Version) provides inducible expression in stem cells. Mouse embryonic stem cells (ES-E14TG2a mES cells) were cotransfected with pTRE3G-ZsGreen1 and pEF1α-Tet3G using Xfect Stem transfection reagent. The stem cells show ZsGreen1 expression only in the presence of Dox (Panel C). Clontech Laboratories, Inc. • www.clontech.com 6 Tet-On® 3G …continued Induce Expression of Two Genes Simultaneously with IR ES Bicistronic Vectors The Tet-On 3G Inducible Expression System (Bicistronic Version) allows the simultaneous expression of two genes of interest using bicistronic vectors. The key element of these vectors is an optimized internal ribosome entry site (IRES) flanked by two MCSs. The inclusion of the IRES permits two genes of interest to be coexpresssed as separate proteins from a single mRNA transcript. Although translation initiation of eukaryotic mRNAs occurs almost exclusively at the 5' cap, the IRES allows ribosomes to bind and initiate translation at a second, internal location. Thus, two proteins are expressed simultaneously from a single bicistronic mRNA transcript (Figures 10 and 11). Select for Clones and Monitor Inducible Expression using Bright Fluorescent Proteins PTRE3G IRES MCS A MCS Using the same IRES technology, we’ve married very tight gene expression control to a bright red fluorescent protein (mCherry) in the Tet-On 3G Inducible Expression System (with mCherry) and a bright green fluorescent protein (ZsGreen1) in the Tet-On 3G Inducible Expression System (with ZsGreen1). If your cells turn red or green after adding Dox, this confirms that your gene has been turned on and that you have selected a high-performing inducible clone (Figure 11). C pA Luciferase expression from MCS II 500,000 mCherry Luciferase 400,000 B Expression of mCherry from MCS I 0 ng/ml 10–1 ng/ml 100 ng/ml RLU 300,000 200,000 101 ng/ml 102 ng/ml 103 ng/ml 100,000 0 0 10–¹ 100 10¹ 10² 10³ ng/ml Figure 10. Co-inducible expression of two genes from a single bicistronic transcript. pTRE3G-IRES expressing both mCherry, from MCS I, and luciferase, from MCS II (Panel A), was cotransfected with pCMV-Tet3G into HeLa cells with increasing levels of Dox. Induced expression of mCherry was monitored by fluorescent microscopy (Panel B) and luciferase expression was measured using a luciferase assay (Panel C). mRNA Doxycycline Ribosome Transcription PTRE 3G mCherry IRES Your gene pA 5' end mCherry Fluorescent protein IRES Your gene AAAAAA Protein of interest Figure 11. Monitor inducible expression and easily screen for inducible clones using bright fluorescent proteins. Clones created using the Tet-On 3G Inducible Expression System (with mCherry) or Tet-On 3G Inducible Expression System (with ZsGreen1) will respectively fluoresce red and green, but only in the presence of Dox. The size (bp) of the fluorescent protein gene is optimal for maximum expression of your GOI from the internal ribosome entry site (IRES). Clontech Laboratories, Inc. • www.clontech.com 7 Tet-On® 3G Mammalian Expression Systems …continued Use Tetracycline Approved Serum for Optimal Results With the greatly increased sensitivity of the Tet-On 3G system, it is more important than ever that the fetal bovine serum you use for your studies is guaranteed to be tetracycline-free (Figure 12). Only Clontech performs actual inducibility tests on a sensitive Tet inducible cell line in order to provide an absolute guarantee that your serum will not affect basal expression in your Tet-On 3G experiments). Each Tet-On 3G system is supplied with 50 ml of our premium Tet-Approved FBS. Visit Visit sit our website for more details! click here… Figure 12. To ensure low background with Tet-On 3G, it is essential to use fetal bovine serum that is functionally tested. Clontech offers four such serum options, including serum that is sourced from the US, Mexico, and Australia, as well as US-sourced serum that is additionally tested for use in mouse embryonic stem cells. References 1. 2. 3. 4. Gossen, M. & Bujard, H. (1992) Proc. Natl. Acad. Sci. USA 89(12):5547–5551. Urlinger, S. et al. (2000) Proc. Natl. Acad. Sci. USA 97(14):7963–7968. Löw, R., Heinz, N., Hampf, M., Bujard, H. & Gossen, M. (2010) BMC Biotechnology 10:81. Zhou, X., Vink, M., Klaver, B., Berkhout, B. & Das, A. T. Optimization of the Tet-On system for regulated gene expression through viral evolution. (2006) Gene Ther. 13(19):1382–1390. Ordering Information Product Size Cat. No. Tet-On 3G Inducible Expression System each 631168 Tet-On 3G Inducible Expression System (EF1α Version) each 631167 Tet-On 3G Inducible Expression System (Bicistronic Version) each 631166 Tet-On 3G Inducible Expression System (with mCherry) each 631165 Tet-On 3G Inducible Expression System (with ZsGreen1) each 631164 Tet System Approved FBS 50 ml 500 ml 631107 631106 Doxycycline 5g 631311 TetR Monoclonal Antibody (Clone 9G9) 40 µg 200 µg 631131 631132 Notice to Purchaser For all licensing information, visit www.clontech.com Clontech Laboratories, Inc. A Takara Bio Company www.clontech.com