solutions
advertisement
Someproblems deal with the allowed valuesof the energyof a hydrogenatom, with the possible
wavelengtls and frequenciesof the radiationemitted when a hydrogenatom makesa transition
from one state to a lower energy state, or with the frequency and wavelength ofradiation that can
be absorbedby a hydrogenatom. The aliowed values ofthe energyare given by
Y/
4.
tru'
I
13.6eV
o"=-a+FF=---7-.
-
wheren is a positiveinteger.Remember
E = 0 meanstheelectronis just freeof theprotonand
hasno kineticenergy.The energyof thephotonis the magnitudeofthe differencein energyof
thetwo statesinvolvedin thekansition:hf:14- E1l.e- Elis positivefor an absorptionevent
andnegativefor an emissionevent.
someprobiemsinvolvethe wavefunctionsfor the electronin a hydrogenatom.Theelectron
movesin three-dimensional
spaceandly42
dv g|esthe probabilityit canbe foundin the
infinitesimalvolumedZ. Theradialprobabilitydensityis givenby p(r) : { mzlyy'zarild,
p(r)dr
givesthe probabiiity that the particlecanbe found in the sphericalshell with inner radiusr and
outerradiusr I dr'.
Questions and Example Problems from Chapter 39
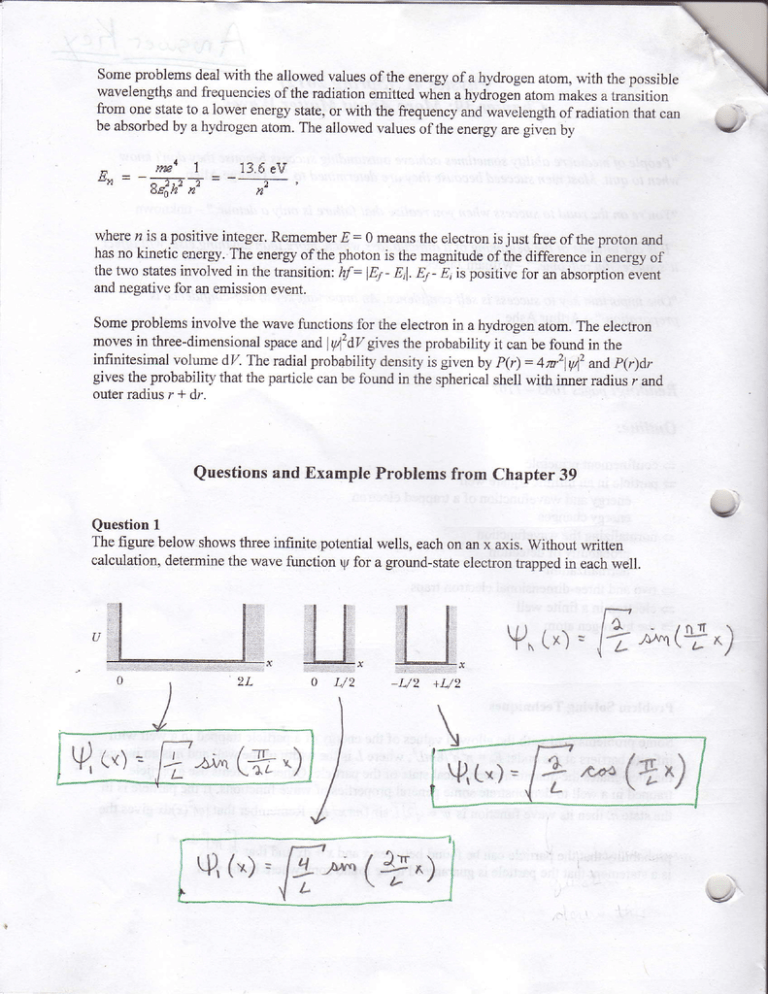
Question1
The figure below showsthreeinfinite potentialwells, eachon an x axis. without written
calculation,determinethe wave function ry for a ground-stateelectrontrappedin eachwel1.
Vn(*) =
l(t) =
I
L
,^ (# -)
tP,(")
13-"
{/-
\l
ff*,e
X.)
\P,t*) =
(+^)
v
I
ProblemI
A proton is confinedto a one-dimensionalinfinite potentialwell 100pm wide. What is its
ground-stateenergy?
rQr= I't,?*,o"?Kr
L= looprn
= loo" lo-'*^
I
\
X"
)
En = (-*;,)
r
, ^
n=))e'3>
""
"^
arta& --+ n = I
g/t-*J
L.
E ,' = --)>
8"q L}
-p.='
(G'ut*lo-zq5'')a
E : 3.2,1* 1d'r5
8(t.t"?* tdt"rg;(roo^!d'\)'
= O. o?'o6q V
Problem2
Aa electronis trappedin a one-dimensionalinfinite potentialwell. (a) What pair of adjacent
energylevels (if any)will havetlree timesthe energydifferencethat existsbetweenlevelsn: 3
andn : 4? @) What pair (if any) will havetwice that energydifference?
/ hl- \I nl
, ..a,^,in#o*,,l--L-'' ,' ____-_>
aA,a^r;*.,te.J"g?
(8.qL',/
fu"r]_ r^"^"+-"A"
= A, nt
ja nrl
|
r.
Xn=
(")
I
)
€^-,
- 2!. -
F, (.*l)'-
,t^rt ,uj-^f- t^*r*
= ( E-)In*r)^
_
Fn*,
'r+t
\ ,.r",,
z
n
'
. E, (n*\)
E, D'
En = 71, (a"*r)
= 3 (Er-trt)
A, (t,.*,)= 3 f a, t"')*-E, (3)"1
'
,- -'l'
r--1 \d.n*'J .L, / E rJ *
: .3l'7F'l
i
, ,.,
.\:i'
o r'-
-;
-
'-"21 *''-.t9^--"*
n=-,lo n'"n
An-t 1="|1
An*
1 Al 2l
!'
/\
' :=
,
l;
f-----
P^*^'W
-Xt/v414 b
b) ,.^,*/"u*.t 4*, - En= E,(ln*l) ='I(tr,-tr"))n:*^
"':
A, (an*r)= t f e, (q')"- (3)"1 * '' [ ?A']
{l
.!
= t1 ---+ | w, nAaa N"I4Aa^'f'^A1 (antt) = if tr, ---" An-n1
zr-n^a W
\ ^ ^^ ".,San^t,L
IA,'x@
I
I
Problem 3
Supposethat ar electrontrappedin a one-dinensionalinfrnite well of width 250 pm is excited
fiom its first excitedstateto jts third exiited srate.(a) [n e]eclron-volts,
what eneigymust be
transfenedto the eiectronfor this quantumjump? Ifthe electronthen de-excitesby emitting
light, (b) what wavelengths can it emit and (c) in which groupings (and orders) can they be
emitted?(d) Show the severalpossibleways the electroncan de-exciteon an energy-level
diagram.
E:
(-n
a
8 r.rL*)
(
t
n\=
( r.a3*ro-']f,..)'
l
''tcz)(15arto''lo)a
6 (q-n" to
n\
(l.us* lo-'*r) nr
En=
( a.oaev) n\
(")
z'pfutali,b
M
p.'rivJ-r,r4}.A,utdo
*-r 6= q
A F= Er- Ar
---+ n='l
=
(6.o3,rv) (,it-2-)
D E ' ??,.a1-V
(u)
hf = AE
6/r=oF -:
P"aarJA
=e
:=:-
\=
\ : lJ- Inm
(.)
'===:
'
4/ ->
\
?---ra l--t I
,l-a3 3 -- |
/i--r3 3-ra a+l
\-=::,= 4 l ' X n $
X = )-5.?n.'\
n=l
.:--:---
- -V- -. .n r n
= 'l -) .Q' -o- e
.= 13.'7nr.
&-q'E, ( t o 1 " v ) ( ' 1 ' - l ' ) = :
= ?.j.tl ..r
q 'L{ -+ h= A.
p 1= ) - r r 6 = |
\/ng
["
fi*r"'lon"
-+ n= 3
n=q
---:=:'
y ^ 1= ] - - s
)=
X = 68'5n',
e--->
ft={
({)
n=3
rr=:.
n=\
Problem 4
A particlb is confinedto the one-dimensionalinfinite potential well ofthe figure belor,. If the
particle is in its first excited state(n : 2), what is its probability of detectionbetween(a) x : 0
and x : 0.25L, (b) x : 0 and x : 0.50L, and (c) x : 0.25 L altd x : L?
,P"c.)=f;(T)
\(r) =i{ **. (li') V"*t*)= YL,.,\\(^"L)*
/'o'L5L
(
(\ 4 )/
\
n,
- e/ ^Anx\ r
-?
/,'tt'n(t)ly
x:o
u = 2"rx/t
Jt=LA"dq
'-r/t
n/t
/,xt\t
du = L/t dr
( /")(Lrn)J,-"'"in
tn/^
'
= * LAx-/q 'r)1
oo
- Y^*4f =
- (t/s'o
A"'r
Y^ntr-+
|
W
=+
TL-'-1
(u) r,--* ttz,,*ry,1!y^hr r*{;*
.
1
\'/. \
probfems
a
f=o.rr.=l-)"
ts
-)W
.x=\Z:;r"oL
I
I
tt
I zt
= l-'/'t=)71
(a; The figurebelowgives'theenergylevelsfor an electrontrappedin iTifri6p-otenrialenergy
well 450 eV deep.if the electronis i,nthen: 3 statgwhatis its kineticenergy?ft) Theelectron
then absorbs500 eV of energyfrom an exlemal source.What is its kinetic energyafterthis
absorption,
assumingthatthe electronmovesto a positionfor which x > L.
_,,-Nonquandzed
-Top
of rvcll
L
/c,)
d,o\)
= }3o<.y'+
{**t-r
U-r-t-V
it
-=
'n/aY. ,D a
":\r
5ooary' =
?f o-,,/ = J{+u)
: )i
V -'1 L,tOe'J
----)
?
J
Problem 6
Ar electronis containedin the rectangularbox of the figure below, with
widths L_ = g00 pm,
Lr= 1600 pm, andr-:400 pm. what is the electron'sglound-stateenergy
in eleciron-voits?
"r-
L nx)n)
I rr.
.
= -6;h' 1": -:---G-{,,
t e"" Zr.
,I
-i vlt.r"i.,p,_t,Tsh
^
l
h,.,..
r)trl
:
Xttt
{\
- f) =
.,]
L
I'
I
\.
:-
q,*r,<,.. ...1",q ft y = Oy
1-:-1-:--::
Lx
:-..t
;_1
i
J
L L.ba - trr-'r f .: )
8(I'tt"lo-::'
t
''
{4oa' io
!:')
F, ,,,j " 4'?5*
ir
Problem 7
3. joeV
iD-'*5.
of widthsL" = L andL, : )y containsan electron.what
r r u q r rmuitiple
nqrulJre
vof
r
"orral
where m is the electron's
, r
3
X.ggul,*
h-l6mI-"
, r,^
s
l
mass,are (a) the
energyof the electron's$ound state,(b) the
energyoritsnrsrexcited,",","i;;#;;#;;'rff
;T::i.43"#f
T#.:,Tf&iitrj
difference between the energies
of its second and third excited states?
(*xs.*) =(*) t*.9,J
an*,n,=
=(*t(f.s)
(a) q,-A,#r"*
(t)
E,,,=(*)
h=\
+^"trrc.^fadd*
--2 nx-l
()Y -- '{
(,r /,) =
E,,^=(;,^)(,.
(.) t^rd)r4r""""t,&J" .--)ll=1, E,,r=(*)
*)
0\%)
^(*)
5*t
=
=
t (-ut')
E.,'.
(rr
(
7,
)
;r-)
ll.l
(l;
,arr-1 ttc'flA,^fi*o --t
l:=j
E,,t
=e;') 1i/')=?(#)-- '
+=-AA = (t)
Vs^t)
Problem 8
(a) What is the wavelengthoflight for the least energeticphoton emittedin the Paschenseriesof
the hydrogenaton spectrumlines? (b) What is the wavelengthofthe serieslimit for the Paschen
series?
'at = (-t:'a"v)
t+'3
hf
(t,-;k
-,
P^*r^r^)r)
a e = (Bl "v)(+ -*-i)
ll::=i
)
(") X^,* r^-4'fi2 4 nr"ra= rl i A E, (t:.{"eV)(+.
*)
=
WV
| 875 "\
\ = ltqo"V.t'cq=- lr-=j=;il
"-"-'I
/.jtl-V
l"
Problem9
A hydrogenalom, initially at rest in the n = 4 quantumstate,undergoesa transition to the ground
state,emitting a photonin tle process.What is the speedof the recoilinghydrogenatom?
?
As,,r'*ili-tr;ro.i';-a>
", fl p,rr-*^rfur.1.,
ldu ..r.'-.c...r,-,rfi+,"1lg E|*
-.t'.*'s",1.,s"-."{Ir'1c,-i J;ou ,. w;-"+"-!"re,,n
}.,1,-;L.zrvou-1*b*r'
Pu
)
{ ,il = a), )*,r^ -{r+,.*1
**e*']'u" ;","r*k.'r"{-{l4rr* ,"&i
"+ .).i\",I',,^-ixl,"d€r^r
-
F$-J*4r"1 "r."J 3"
jo$t:, ,!.ntr*r*
rw,-,,,-e*-.$r^,.,+
'---'---= \/,^ = 'y.
ffl*Vn
l*,u*-,
izr{-e.."J1'a
rf
1}*t-lJ4"r'"'i-o *''*-n-'X -'t-b-\a : i-l i = Er-F,
-P.+,iu*c.f!
"''',
6r};fuo'r
f**.*)
= lt.-t5e-J
1
i-
D*V* ' l'+/- =
( t:.ts.v)
-:
' 'tl
rr., C
(r: ts.v) ( t'y"';:t;''V.u)
( 1 . r , 1 2 , , t € ' ' ' u 2( ): ' o ' ) t > x o>f )
t'---------------
=
------u
ii { r', 4')",f5 i
i,!
l, b lr, lo
Kdo
Problem 10
(a) Find, usiug the energy-level diagram of the figue below, the quanturn numbers
corresponding to a transition in which the wavelength of the emitted radiation is 121.6 nrn. (b\
To whal seriesdoesthis tmnsmissionbelons?
Nonquantized
E=hf .= \-/\
-2.0
.
4.(l
6
;
-6.0
.i
-B,0
E=
I At-fO eV.om
I )"1.L orq
H
'
-10.0
// ..- :-'
l[Illt_
E = lO'l.-V
(
-14.0
-l
i6;;-;il
/r/^t
-lrt Fr^J "$ f"a*'-"^-'t^
{u; lt/.d".-^t/-'r"l
(")
hi= /\E = Etn*-Eu*
lD.t".v -13-4n\
- 3.'1-V -- - 13't"eV
-..-+
E
n'
flt=
l?'GeV =tl
3-rl e V
Problem1I
A hydrogen,atomin a statehavinga binding energy(the energyrequiredto removean electron)
of 0.85eV makesa transitionto a statewith an excitationenergy(the differencebetweenthe
e,nergyof the stateandthat of the ground state)of 10.2eV. (a) Wlat is the energyof the photon
emittedasa result ofthe transition?(b) Identifi this traasition,usrngthe energyJeveldiagramof
the figure below.
0-
*L
= -D-85-V
Er=
-)3.t"eV+ lo.)eV
4.0
-6.0.
g,
Ee
_-8.0'
-10.0.
- (-3.'i
-12.0
"v)
-140
( b)
F, +/qll""
bt*r"
) ^4,o^^cr+.r /040 Jiat
h
Azr.o
t)
D=?.
---> A F = En - Ae
.ttbtet
bpl**,
- tzss - tt::9
/\a
n).
- 13.t"'v
=-..fi\
D=4
/.-\
+ t ' 1 z|, V
e'"bqv
-,r.u'l^,oaa
^ pr"b'l
=
("
= - 1 3 . . -e- .-. -V- ( - 1 3 , 6e u )
n'
aa
l'55 e/
= 1..55- V ---->
13,G4V
na
= -o.85ev





