Tenyear results of galvanic sacrificial anodes in steel reinforced
advertisement

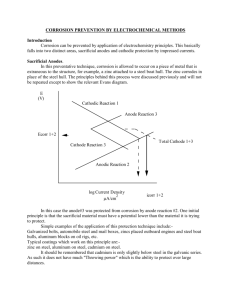
98 DOI: 10.1002/maco.201005707 Materials and Corrosion 2011, 62, No. 2 Ten-year results of galvanic sacrificial anodes in steel reinforced concrete G. Sergi* Zinc sacrificial anodes have been included in patch repairs to steel reinforced concrete structural elements suffering from corrosion since the mid-1990s. A number of these anode-containing repairs have been monitored with time. One of the first monitored sites was of a locally repaired cross beam of a bridge structure in Leicester, UK, which has now completed 10 years since its original repair and anode installation. This paper reviews the performance of the anodes installed at the Leicester site in terms of anode current output and steel reinforcement polarisation and corrosion rate over the period. It also presents results of analysis of recovered anodes exposed for 10 years which still show electrolyte continuity, uniform consumption of the zinc and coherent encasing mortar. The knowledge gained from the 10 year results has enabled the development of new, higher current output anodes, which are now trialled in this and other sites. 1 Introduction Chloride induced corrosion of steel reinforcement in concrete structural elements is a major problem in many countries. Chlorides can be introduced into the concrete via deicing salts or seawater. This leads to localised breakdown of the normally passive steel reinforcement in the form of pitting corrosion. Patch repairs of only the damaged concrete are rarely, if ever, successful where chlorides are present in sufficient concentration in the remaining concrete. Under such conditions, treatment of the symptoms merely serves to send the corrosion cell into areas adjacent to those that have been repaired. This phenomenon is known as the incipient anode or ring effect [1–3]. Consequently, traditional repair techniques, where chlorides are present above certain levels, are only likely to be effective for a limited period after which time further repairs will be necessary. It is essential that some form of intentional ‘cathodic prevention’ be reinstated within the patch repair region so that the adjacent areas remain cathodic and corrosion initiation is prevented. This can be accomplished by embedding sacrificial anodes around the perimeter of the repair patch. Galvanic anodes of an appropriate design were developed in the late 1990s. Their success under laboratory conditions and in small-scale controlled trials was demonstrated over a period of about a year [1, 2]. The puck-like anode was produced from zinc metal encased in a specially formulated porous cementitious G. Sergi Vector Corrosion Technologies Ltd., 3 Bodmin Close, Park Hall, Walsall WS5 3HZ (UK) E-mail: georges@vector-corrosion.com ß 2011 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim mortar (Fig. 1) saturated with lithium hydroxide (pH > 14.5). Such an environment, with a reservoir of excess LiOH maintaining a constantly high pH, was shown to sustain the zinc in an active condition producing soluble zinc corrosion products that do not stifle the corrosion process of the metal [1]. Several trials were set up as part of the repair regime undertaken for the rehabilitation of structural elements over the last 10 years both in the UK and abroad. The anodes were used for two types of application, viz., around the perimeter of patch repairs in a cathodic prevention mode, and in a grid configuration in susceptible areas of reinforced concrete in a corrosion control mode. Some of the results have been reported elsewhere [3]. Current densities of the order of 0.8–10 mA/m2 of steel surface were recorded depending on the type of application. The oldest site trial, at a bridge in Leicester, UK (Fig. 2), has completed 10 years of life this year, a milestone, as the anodes were designed for a minimum life of 10 years. It is the results of this trial that are reported in this publication. 2 Setting up of the trial As verification for the performance of the anodes in actual structures, a total of 12 commercial anodes were installed in an otherwise conventional patch repair [4] on spalled and cracked areas of a beam section of a bridge in Leicester, UK (Fig. 2). The trial was set on the soffit of a section of the beam between Columns 6 and 7 on the west pier (Fig. 3). The performance of these anodes was monitored with time. This formed part of a repair scheme for the whole bridge which contained two abutments and two piers each consisting of a long cross-beam sitting on eight columns. The overall repair system was made wileyonlinelibrary.com www.matcorr.com Materials and Corrosion 2011, 62, No. 2 Figure 1. Puck-type anode used for the trial Figure 2. Installation of anodes within the repaired area of a beam showing also control box and wiring up of patch repairs containing anodes around their perimeter and included impressed current cathodic protection with discrete anodes at the more deteriorated areas at the top of the abutments. Galvanic sacrificial anodes in steel reinforced concrete The extent of deterioration of the beam section that was chosen for the trial is summarised in Figs. 4 and 5 which show the chloride contamination at increasing depths and the potential map prior to repair. The chloride concentration at the depth of the steel was over 2% by weight of cement in location A, close to the most negative recorded potential within the area. In location B, the chloride concentration was just below 1%. Both concentrations are overall higher than the range of chlorides found in this particular part of the pier outside the area of repair and the mean for the west pier but lower than the maximum concentration found in the pier (Fig. 4). The potential map suggested the presence of corrosion activity of the steel reinforcement which was accompanied by some cracking and delamination. The range of potentials within the test area is similar to the whole west pier (Fig. 6), a significant proportion of the potentials indicating the presence of some corrosion activity. The concrete was broken out to behind the steel and to beyond any corroding steel as shown in Figs. 2 and 3. No attempt was made to ensure that chloride contaminated concrete areas were removed, the level of chloride in the adjacent unremoved concrete and at depth beyond the steel was known to be significant, as suggested by Fig. 4. These are classic conditions for the formation of new anodic sites at the periphery of a conventionally repaired area causing corrosion of the steel and cracking of the concrete within a few years [1, 2]. A total of eight anodes were inserted around the perimeter of the left hand repair area and four anodes were inserted in the right hand area at between 600 and 700 mm centres (Figs. 2 and 3). The anodes were specially adapted to enable monitoring. A single wire from each anode was connected to a control box so that connection could be made individually to the steel reinforcement via the box. All other similarly cracked, delaminated or spalled areas of the pier were likewise repaired with anodes positioned at approximately 600–700 mm centres around the perimeter of each area. These were connected directly to the steel reinforcement using the four tie wires available [4] (Fig. 1). Figure 3. Schematic of the repaired area of the beam soffit www.matcorr.com ß 2011 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim 99 Sergi Materials and Corrosion 2011, 62, No. 2 Figure 4. Chloride concentration profiles at positions A and B within the subsequently repaired area of the beam compared to around the beam (no. 1–4) and the maximum and mean for the whole west pier prior to repair Monitoring of the 12 anodes was by a combination of current output measurements for each installed anode and, on occasions, a depolarisation potential over 4 or 24 h periods after disconnection of the anodes. A further measurement was the ‘instant-on’ current output of each anode following reconnection to the steel. Monitoring started in April 1999. 3 Results and discussion The total estimated current output of each anode could be converted to a charge as shown in Table 1. Using Faraday’s law, and assuming an efficiency and utilisation rate for the metal, the total consumption of the zinc metal could be estimated. For an efficiency of around 85% the level of consumed metal is as shown in Fig. 8. By simple extrapolation, the service life of each anode can be determined. According to these results, a range between 24 and 37 years service life can be achieved for these anodes with 60 g zinc mass. The 10 year results of the current output of each anode are presented in Fig. 7. They indicate a variable current depending on the moisture content in the concrete but primarily on temperature (see also next section). For example, the same anode could generate up to 400–600 mA of current during hot periods and less than 100 mA during cold spells. Corrosion of the steel is expected to have similarly varying corrosion rates so that the current output of the anodes is thought to be self-regulating, producing higher levels when the steel is corroding most. 0 -100-0 -200--100 -300--200 0.5 -400--300 0 0.5 1 1.5 2 Grid (m) 100 1 2.5 Grid (m) Figure 5. Potential map of the left repaired area of the beam prior to repair (mV vs. Cu/CuSO4) ß 2011 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim Figure 6. Range of potential values recorded within the area of the soffit of the beam chosen for repair compared to all the values recorded over the whole west pier www.matcorr.com Materials and Corrosion 2011, 62, No. 2 Galvanic sacrificial anodes in steel reinforced concrete Figure 7. Current output of the 12 individual anodes and the mean output with time Confirmation of the approximate efficiency was provided by assessing removed anodes from an adjacent patch repair in the west pier (Fig. 9). These removed anodes were installed 10 years ago and the exposure conditions were similar to the 12 monitored anodes. Exposure of the zinc core revealed a thickness of zinc-rich pseudomorphic corrosion product at the interface of the zinc and encasing mortar. No more than 25–30% of the original zinc metal was lost, a level within the range calculated for the 12 monitored anodes (Fig. 8), suggesting that the assumed efficiency of 85% is likely to be a fair estimate. There was evidence also that the pores of the encasing mortar extending several millimetres away from the zinc/mortar interface were partly filled with white zinc oxide and zinc hydroxide corrosion products. This supports the assertion that the zinc corrosion product remains soluble, owing to the pH of the pore solution exceeding 14 as a result of saturated solution of lithium hydroxide present in the encasing mortar, and can travel through the pores before super-saturation and precipitation occurs. The porosity of the encasing mortar is deliberately designed to be high enough to accommodate this kind of corrosion product movement. The mechanism ensures that no stresses and no expansive forces build up around the zinc core and cracks are avoided. It also allows continual exposure of the zinc substrate to the alkali pore solution ensuring its high activity. The level of depolarisation was recorded at 12 points within the repaired area and at 8 points outside the repair. In the repaired area this was a 3 4 grid at 500 mm intervals along the length of the beam and 250 mm intervals across the beam but away from Table 1. Total charge produced by each individual anode in 10 years of exposure Anode number Charge (coulombs) Anode number Charge (coulombs) 63 141 55 696 46 621 41 135 61 228 60 825 7 8 9 10 11 12 Mean 65 194 43 619 59 459 56 333 57 205 48 989 54 954 1 2 3 4 5 6 www.matcorr.com Figure 8. Approximate amount of zinc consumed based on the charge produced and 85% efficiency the anodes. Outside the repair, the points were along two lines on the vertical face of the beam at 50 and 300 mm from the edge of the repair (Fig. 3). The values recorded were the difference between the potential at the nodes whilst the anodes were connected (not the instant off potential) and the depolarised potential at the same nodes 4 h after disconnection of all the anodes (24 h for the 3400 day results). The actual values are tabulated in Table 2. The values are very low initially but approach or exceed 100 mV after 9 years (3400 days) of polarisation of the steel. It is interesting to note that the level of depolarisation tends to be higher outside the repaired area and appears to be still significant up to 300 mm away from the edge of the repair. Systems such as this are designed to cathodically prevent the onset of corrosion of the reinforcement and not to control existing corrosion as is the case in cathodic protection systems. As such, a much lower current density (0.2–2 mA/m2) is necessary for cathodic prevention, as reported by Bertolini et al. [5] and Pedeferri [6] and adapted in the European Standard EN 12696:2000 [7]. Estimation of the steel surface area within the repaired and affected adjacent area shows that the mean current density ranged between 0.6 and 3.0 mA/m2 with an overall mean of around 1.4 mA/m2, generally within the suggested range for cathodic prevention. Considering that the depolarisation level from the monitored site increased substantially with time, indicating a good level of protection of the steel reinforcement, and that after 10 years life the patch repair is still intact and operating well, it is reasonable to suggest that the 100 mV depolarisation criterion is not applicable for this type of cathodic prevention system, especially early on. Other criteria for determining the effectiveness of a cathodic prevention system may be necessary. Monitoring the depolarised potential of the steel in the vicinity of the repair with time may be a more effective way of determining the effectiveness of the system. Figure 10, showing the mean depolarised potential with time both within and outside the repaired area, indicates this. It is clear that the recorded mean potential is continuously moving to a more noble level with time indicating increasing passivation of the steel. The corrosion current density of the steel reinforcement, a direct measure of the state of the steel, is probably the best parameter available for monitoring the performance of the system. This was simply estimated using Equation (1) which ß 2011 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim 101 Sergi Materials and Corrosion 2011, 62, No. 2 Figure 9. (a) Removed 10-year-old anode showing corrosion product around zinc and intact encasing mortar. (b) Zinc core with part of the corrosion product broken off exposing the zinc substrate requires only measurement of the applied current density and equivalent depolarisation potential. icorr ¼ iappl =½expð2:3h=bc Þ expð2:3h=ba Þ (1) where icorr is the corrosion current density, iappl the applied current density, h the observed potential shift (polarisation potential), bc the cathodic Tafel slope (assumed as 120 mV) and ba is the anodic Tafel slope (assumed as 60 mV). The corrosion current density was shown to diminish with time. It averaged around 0.7 mA/m2 up to day 112 and reduced substantially to 0.2 mA/m2 after 9 years. These levels are considerably lower than 1–2 mA/m2 which is assumed to be the limit above which corrosion becomes significant. Another interesting parameter that could be useful as a criterion for establishing the continued effectiveness of galvanic anodes is the current output at switch on. In this case, it was measured five seconds after reconnecting each individual anode to the steel while all other anodes were disconnected following the 4 or 24 h depolarisation period. The mean current appears to have increased with time right up to 10 years (Fig. 11). The magnitude of this current is likely to be related to the potential difference between the zinc anode and the steel, which may be thought of as Table 2. Mean depolarisation values at 4 h (24 h at 3400 days) No. of days from switch-on 21 41 50 112 3400 West vertical face of beam at shown distance from edge of repair (mV) Beam soffit within the repaired area, midway between anodes (mV) 50 mm 300 mm 56 27 22 24 95 58 47 55 48 184 56 31 28 11 Not determined ß 2011 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim a ‘drive potential’. The zinc potential was not measured but a plot of the ‘instant-on’ current versus the mean potential of the steel within and around the repaired area just before switch-on, a parameter related to the ‘drive potential’ if the potential of the zinc is assumed to be reasonably stable, suggests a good correlation (Fig. 12). Both Figs. 11 and 12 also suggest that the anodes are still able to perform to a very high level after 10 years of operation. The generally decreasing current output of the anodes shown in Fig. 7 may thus be related primarily to the changing conditions of the concrete, as drying of the pier has been occurring owing to the repair of leaking joints, and possible changes to the steel concrete interface such as increased alkalinity and enhancement of the passive oxide film or localised deposition of solid phases owing to the continuous polarisation of the steel [8]. 4 New developments Knowledge gained from this and other trials [3] enhanced by additional research has enabled the further development of 300mm outside repair Steel potential (mV vs Cu/CuSO4) 102 50mm outside repair Within repair 100 0 -100 -200 -300 -400 10 100 1000 10000 Time (days) Figure 10. Mean depolarised steel potentials with time (4 or 24 h after disconnection of the anodes) www.matcorr.com Materials and Corrosion 2011, 62, No. 2 Galvanic sacrificial anodes in steel reinforced concrete 'Instant-on' Current (mA) 2000 1500 1000 500 0 10 100 1000 10000 Time (days) Figure 11. Variation of mean current output with time of anodes at switch-on after depolarisation Figure 13. Cross-sectional detail of the abutment rehabilitation system concrete was removed; long lengths of the anode were connected to the existing reinforcing steel and embedded in a new concrete layer along with additional epoxy coated reinforcement down the whole face of the abutment wall. The purpose of the anode network was to protect the existing steel from chloride-induced corrosion allowing un-cracked chloride-contaminated concrete to remain in place and thus reduce concrete breakout. The cross sectional configuration of the repaired abutment wall and adjoining structural elements are shown in Fig. 13. The current output, shown in Fig. 14, is seen to be strongly related to temperature. Its magnitude varied considerably on an annual basis with temperature but the mean current density has been gradually reducing year by year. After an initial level of over 35 mA/m2 of steel area in the first few days, it averaged over 8 mA/m2 during the first year lowering gradually to around 5 mA/ m2 in the fourth year. These levels of current density are within the design limits of 2–20 mA/m2 of steel area for cathodic protection as specified in EN 12696:2000 [7]. Current densities in impressed current cathodic protection systems are also normally reduced with age as the steel becomes easier to polarise. Depolarisation levels were measured to be well in excess of 100 mV as specified in the same standard, suggesting that the galvanic system was deemed to satisfy the criteria for cathodic protection of steel reinforcement. 100 60 120 0 -100 -200 -300 50 1 00 1 00 0 100 00 'Instant-on' current (mA) Figure 12. Relationship between mean ‘instant-on’ current and mean rest potential of steel within and around the repair area www.matcorr.com Temperature 40 80 35 30 60 25 20 40 15 10 5 -400 100 45 20 Temperature, degree F 55 Galvanic Current, mA Rest Potential (mV vs Cu/CuSO4) galvanic anodes. Single anodes with modified geometry and encasing mortar composition have been developed with double and four times the current output capability for use in more severe conditions. Some of these have now been installed in the same UK bridge as a new trial, following removal of a number of original anodes for analysis. The new repaired area contains three ‘double-output’ anodes and seven ‘quadruple-output’ anodes at a maximum spacing of 300 mm. Chloride levels in the adjacent undamaged concrete was found to be in the range 1.0–2.6% chloride by weight of cement. Normally, this highly contaminated concrete should be removed but it provides an opportunity to test the capability of the improved anodes. Monitoring is at its infancy but very early results show a mean ‘instant-on’ current of 1450 mA per ‘double-output’ anode and 2620 mA per ‘quadruple-output’ anode, both considerably higher than the current of the original anodes found to be of the order of 800–1000 mA at the equivalent time. Other configurations of galvanic anodes have also been developed for more global corrosion control methodologies. One such configuration is the system installed at a bridge abutment in Ohio, USA. The abutment had been contaminated with chlorides causing localised corrosion of the reinforcing steel. As part of the rehabilitation, which also included enlargement and strengthening of the abutment, the cracked and spalled Current 0 0 May-Aug-Oct- Jan- Apr- Jul- Oct- Jan- Apr- Jul- Oct- Jan- Apr- Jul- Oct- Jan- Apr05 05 05 06 06 06 06 07 07 07 07 08 08 08 08 09 09 Date Figure 14. Current output of anode system and its relationship to temperature ß 2011 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim 103 104 Sergi 5 Conclusions Zinc galvanic anodes, activated by a lithium hydroxide saturated mortar, were shown to be successful in providing adequate cathodic current to the steel reinforcement around the periphery of a patch repair for a period of 10 years. This ensured that no incipient anodes were formed on the steel adjacent to the repaired area and the repair as a whole remained intact and free from corrosion of the steel reinforcement. Extrapolation of the results also showed that a service life of between 24 and 37 years can be expected from these 60 g zinc anodes. Depolarisation levels of the steel reinforcement after disconnection of the anodes for periods of either 4 or 24 h showed a diminishing trend over the first 112 days, rarely exceeding 50 mV around the periphery of the repair, but then increased to over 100 mV after 9 years. The 100 mV depolarisation criterion, which applies to cathodic protection systems, is unlikely therefore to apply for cathodic prevention systems of this type. An alternative more realistic criterion should be developed. It is suggested that the change in the rest potential of the steel, following periods of depolarisation over a constant time (4 or 24 h), be considered as a criterion for establishing the performance of a corrosion prevention system. This can be aided by estimation of the corrosion current density of the steel from knowledge of the depolarised potential and the equivalent applied current density. In this particular case, it was seen that the steel rest potential gradually moved in a positive direction while the corrosion current density diminished to a very low level signifying improved passivity of the steel. Although the current output followed an overall decreasing trend, the driving power of each anode did not show any evidence of diminishing. To the contrary, the current output at switch-on, following a period of depolarisation, was seen to increase with time, possibly because the potential of the steel gradually moved Materials and Corrosion 2011, 62, No. 2 in the positive direction thus increasing the ‘drive voltage’ between the anode and the steel. Lessons learned from this and other trials and from further research have enabled the production of enhanced performance anodes using a better surface area to volume ratio and improved chemical composition of the encasing mortar. Anodes with double or quadruple the current output capability have been used for a new trial at the same site. Early results confirm their higher capacity. The technology was shown to be very flexible and by utilising a distributed current-type anode set-up consisting of long anodes affixed along the steel reinforcement, it was possible to provide depolarisation levels exceeding 100 mV, and to achieve current densities compatible to conventional impressed current cathodic protection systems. 6 References [1] G. Sergi, C. L. Page, presented at EUROCORR’99, Aachen, Germany, August 29–September, 1999. [2] C. L. Page, G. Sergi, J. Mater. Civil Eng. Sp. Issue, February 2000, 8. [3] G. Sergi, D. Simpson, J. Potter, presented at EUROCORR 2008, Edinburgh, UK, 2008. [4] D. Whitmore, Field Guide to Concrete Repair Application Procedures ACI RAP Bulletin 8, 2005. [5] L. Bertolini, F. Bolzoni, A. Cigada, T. Pastore, P. Pedeferri, Corros. Sci. 1993, 35, 1633. [6] P. Pedeferri, Constr. Build. Mater. 1996, 10(5), 391. [7] British Standard BS EN 12696:2000, 2000. [8] G. K. Glass, B. Reddy, in: R. Weydert (Ed.), COST 521: Final Reports, Luxembourg University of Applied Sciences, Luxembourg, 2002, p. 227. (Received: March 6, 2010) (Accepted: April 8, 2010) ß 2011 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim W5707 www.matcorr.com