Gases: Heavier or Lighter Than Air? Density Explained
advertisement
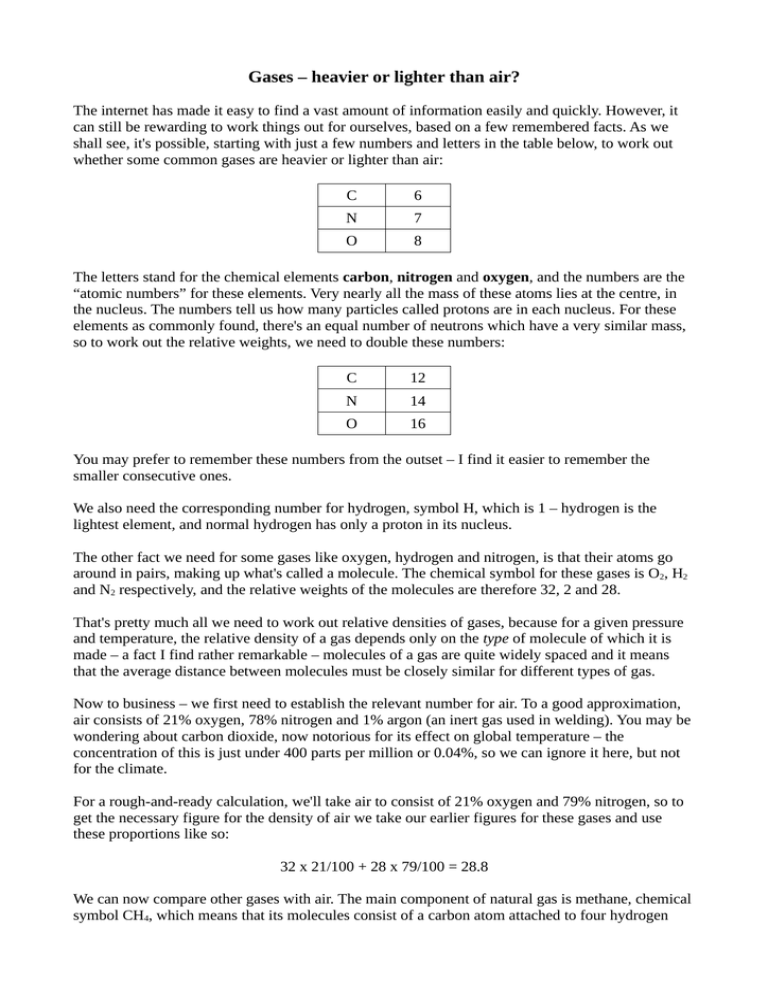
Gases – heavier or lighter than air? The internet has made it easy to find a vast amount of information easily and quickly. However, it can still be rewarding to work things out for ourselves, based on a few remembered facts. As we shall see, it's possible, starting with just a few numbers and letters in the table below, to work out whether some common gases are heavier or lighter than air: C 6 N 7 O 8 The letters stand for the chemical elements carbon, nitrogen and oxygen, and the numbers are the “atomic numbers” for these elements. Very nearly all the mass of these atoms lies at the centre, in the nucleus. The numbers tell us how many particles called protons are in each nucleus. For these elements as commonly found, there's an equal number of neutrons which have a very similar mass, so to work out the relative weights, we need to double these numbers: C 12 N 14 O 16 You may prefer to remember these numbers from the outset – I find it easier to remember the smaller consecutive ones. We also need the corresponding number for hydrogen, symbol H, which is 1 – hydrogen is the lightest element, and normal hydrogen has only a proton in its nucleus. The other fact we need for some gases like oxygen, hydrogen and nitrogen, is that their atoms go around in pairs, making up what's called a molecule. The chemical symbol for these gases is O2, H2 and N2 respectively, and the relative weights of the molecules are therefore 32, 2 and 28. That's pretty much all we need to work out relative densities of gases, because for a given pressure and temperature, the relative density of a gas depends only on the type of molecule of which it is made – a fact I find rather remarkable – molecules of a gas are quite widely spaced and it means that the average distance between molecules must be closely similar for different types of gas. Now to business – we first need to establish the relevant number for air. To a good approximation, air consists of 21% oxygen, 78% nitrogen and 1% argon (an inert gas used in welding). You may be wondering about carbon dioxide, now notorious for its effect on global temperature – the concentration of this is just under 400 parts per million or 0.04%, so we can ignore it here, but not for the climate. For a rough-and-ready calculation, we'll take air to consist of 21% oxygen and 79% nitrogen, so to get the necessary figure for the density of air we take our earlier figures for these gases and use these proportions like so: 32 x 21/100 + 28 x 79/100 = 28.8 We can now compare other gases with air. The main component of natural gas is methane, chemical symbol CH4, which means that its molecules consist of a carbon atom attached to four hydrogen atoms, giving a total weight of 12 + 4 = 16. This is considerably less than the figure for air – methane is lighter than air. We can find its density relative to air by dividing its weight figure by that of air: 16/28.8 = 0.56, a number familiar to many gas engineers. Carbon dioxide is CO2, one atom of carbon attached to two of oxygen, weight 12 + 2 x 16 =12 + 32 = 44, considerably heavier than air. The toxic gas carbon monoxide is CO, weight 12 + 16 = 28, very slightly less dense than air (so place carbon monoxide detectors relatively close to the ceiling of a room). Butane has the chemical formula C4H10, weight 12 x 4 + 10 = 58, much heavier than air. Propane is C3H8, weight 12 x 3 + 8 = 44, the same as carbon dioxide and also heavier than air. Butane and propane are the main constituents of liquified petroleum gas (LPG), which is why LPG in a basement or trench can be a thoroughly bad idea (if there's a leak it'll stay there, building up in concentration). Given that air is a mixture mostly of two gases, we might wonder why the denser oxygen doesn't settle below the lighter nitrogen. It seems the atmosphere is being continually stirred, preventing this from happening. Perhaps if we sealed some air in a vertical tube we might find over time that the gases separated out. End of document






