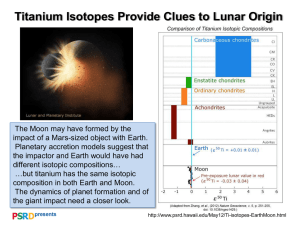
Ten years of experimental animal isotopic ecology
advertisement

Functional Ecology 2009, 23, 17–26 doi: 10.1111/j.1365-2435.2008.01529.x NUTRITIONAL ECOLOGY Blackwell Publishing Ltd Ten years of experimental animal isotopic ecology SIA in animal ecology Nathan Wolf*, Scott A. Carleton and Carlos Martínez del Rio Department of Zoology and Physiology, University of Wyoming, Laramie, WY 82071-3166, USA Summary 1. Ten years ago Gannes et al. (1997, Stable isotopes in animal ecology: assumptions, caveats, and a call for laboratory experiments. Ecology, 78, 1271–1276, 1998) identified four major areas requiring further research in experimental animal isotopic ecology: (i) the dynamics of isotopic incorporation, (ii) mixing models, (iii) the problem of routing, and (iv) trophic discrimination factors. 2. Differences in isotopic incorporation rates among tissues seem to be explained by variation in protein turnover. The application of multi-compartment models to isotopic incorporation data has revealed that different inferences can be derived between these and one-compartment models. 3. A variety of mixing models of varying degrees of complexity and realism are used to find the contribution of isotopic sources to the elements in an organism’s tissues. The use of these models demands the use of tissue to diet discrimination factors that are rarely measured experimentally. 4. Mixing models assume that assimilated nutrients are disassembled into their elemental components and that these elements are reassembled into biomolecules. This assumption is unrealistic as macromolecules are routed differentially into tissues. Isotopic routing is an area that isotopic ecologists have neglected in their experimental and modelling research. 5. Isotopic ecologists are just beginning to understand why 15N biomagnifies along trophic chains, and to explore the factors that determine the degree of 15N biomagnification. We review the hypotheses that explain why 15N biomagnifies up trophic chains. 6. The use of compound-specific isotopic analyses is opening new fruitful areas of research at the intersection of nutritional and isotopic ecology. Key-words: δ13C, δ15N, experimental isotopic ecology, stable isotopes, trophic ecology Introduction Over the last 10–15 years, animal ecologists have embraced stable isotope analysis (SIA). During this period, the applications of SIA to the study of animals has grown rapidly and ecologists have applied SIA to all areas of animal ecology ranging from paleoecology to ecosystem ecology, passing through physiological and population ecology (Hobson & Wassenaar 1999; Martínez del Rio & Wolf 2005; Koch 2007). The successful adoption of SIA by animal ecologists is the result of (i) technological progress, (ii) large observational data sets, (iii) experimental research, and (iv) the development of theoretical models. 10 years ago, Gannes et al. (1997, 1998) predicted that SIA would grow rapidly and called for laboratory experiments (Gannes et al. 1997). Here we will review the experimental studies that have taken place 10 years after Gannes et al.’s (1997) call for more experimentation. Our primary focus will not be the many insights that ecologists have gained using stable isotopes. Instead, we will focus on (i) areas in which further experimentation is still needed, *Correspondence author. E-mail: nwolf@uwyo.edu (ii) describe models that ecologists use to interpret experimental results, and (iii) identify areas in which theoretical research and development are still needed. Gannes et al. (1997, 1998) proposed that the successful application of SIA to animal ecology hinged on our knowledge of how rapidly and faithfully animals incorporate the isotopic composition of their food. They identified four areas in which experimental work was needed: (i) the dynamics of isotopic incorporation, (ii) mixing models, (iii) the problem of routing, and (iv) trophic discrimination factors. These areas are the organizing foci of this review. In a final section we will identify novel research themes that we believe are ripe for exploration. We begin our review by justifying why a review on the use of stable isotopes has a place in an issue of Functional Ecology devoted to nutritional ecology. ISOTOPIC ECOLOGY AS AN APPLICATION OF NUTRITIONAL ECOLOGY Nutritional ecology investigates the evolutionary causes and ecological consequences of how animals acquire and process resources (Karasov & Martinez del Rio 2007). George © 2009 The Authors. Journal compilation © 2009 British Ecological Society 18 N. Wolf et al. Bartholomew (1964) wrote that ‘Every level of biological organization finds its mechanism at lower levels of biological organization and its significance at higher levels of biological organization’. We believe that isotopic ecology receives its meaning from its relevance in population, community, and ecosystem ecology, and has its mechanistic foundation in nutritional ecology. We will attempt to establish clear connections between patterns that ecologists use and their known or possible physiological causes. Dynamics of isotopic incorporation IMPORTANCE TO ECOLOGISTS Isotopic ecologists should be interested in the time-scale of the incorporation of the isotopic signature into an animal’s tissues because this information determines the time window through which they can perceive the course of diet changes in an animal (Dalerum & Angerbjörn 2005; Newsome et al. 2007). By sampling different types of tissues in a single individual, SIA allows exploration of how animals use resources over a variety of temporal scales (reviewed by Phillips & Eldridge 2006). In vertebrates, some tissues, such as liver and plasma proteins have high rates of isotopic incorporation, and their isotopic composition reflects integration of recent dietary inputs. Others, such as bone collagen, exhibit low incorporation rates, and their isotopic composition reflects integration over longer time periods (Dalerum & Angerbjörn 2005 and references therein). Some tissues (e.g. feathers, hair, and shells) are deposited in discrete intervals, remain inert, and retain the isotopic composition of resources incorporated while they were manufactured (Bowen et al. 2005). ONE COMPARTMENT, FIRST-ORDER MODELS The time course of isotopic incorporation is determined experimentally. A group of animals whose tissues have reached equilibrium with one diet are shifted to another diet with a different isotopic composition (Martínez del Rio & AndersonSprecher 2008). The relationship between the composition of animal’s tissues (δX(t)tissue, where X is an isotope) and time (t) has been traditionally described by exponential functions of the form δXtissue(t) = a + be−ct, where a, b, and c are estimated empirically (Bearhop et al., 2002 and references therein). This equation can be written, perhaps more intuitively, as δXtissue(t) = δX∞ − (δX∞ − δXtissue(0))e−λt, eqn 1 where a = δX ∞, b = −( δX∞ − δX tissue(0)), and c = λ. Eqn 1 represents the behaviour of a well-mixed, one-compartment system with first order kinetics (Martínez del Rio & Wolf 2005; Olive et al. 2003). The average residence time of an element in systems described by eqn 1 equals 1/λ and the median residence time (or half-life, t1/2) equals Ln (2)/λ. (Μartínez del Rio & Anderson-Sprecher 2008). Although we contend that using eqn 1 in all cases is incorrect (see Cerling et al. 2007a), its application has been profitable. WHY THERE ARE DIFFERENCES IN ISOTOPIC INCORPORATION AMONG ANIMALS? The rate at which animals incorporate the isotopic signal of their food differs among organisms and tissues. The factors that have been recognized (or hypothesized) to influence incorporation rate are catabolism (protein turnover), growth and body mass (mb). Carleton & Martínez del Rio (2005) predicted that λ should be proportional to mb−1/4, and a data set on the rate of 13C incorporation into the red blood cells of several bird species verified their prediction. This result suggests that isotopic ecologists may not use incorporation data of an animal to infer the incorporation rate of another of a different size. However, an animal’s body size is not the only determinant of the rate at which its tissues incorporate the isotopic composition of diet. The value of λ is determined by both growth and by catabolic turnover (Fry & Arnold 1982). Hesslein et al. (1993) proposed that the value of λ equals the sum of fractional net growth kg (kg = mb−1[dmb /dt]) and catabolic turnover kd (λ = kg + kd). If isotopic incorporation can be described adequately by eqn 1, we can summarize the effects of growth and catabolism on λ as follows: λ= 1 ⎛ dmb ⎞ θ ⎜ ⎟ + αmb mb ⎝ dt ⎠ eqn 2 This equation states that the fractional rate of isotopic incorporation equals the sum of fractional growth rate and the allometric effect of body size on catabolic turnover. The parameters α and θ are empirically derived constants. We speculate that the value of θ approximately equals −0·25 (Carleton & Martínez del Rio 2005). Because temperature has a profound effect on all metabolic processes (Gillooly et al. 2001), we expect the magnitude of the allometric term to depend on temperature (Witting et al. 2004) and differ between endotherms and ectotherms. Although the predictions embodied in eqn 2 have not been tested quantitatively, available data is consistent with them. Isotopic incorporation is rapid in fast growing ectotherms. (Jardine et al. 2004; Suzuki et al. 2005 and references therein, McIntyre & Flecker 2006; Reich et al. 2008), and the contribution of growth to λ in the tissues of ectotherms is high (from 30% to 100%). In contrast, MacAvoy et al. (2005) found that growth accounted for only c. 10% of the rate of incorporation of carbon and nitrogen in adult mice. Tieszen et al. (1983) warned about the ‘... important complication ... that each tissue ... can be expected to have an isotopic memory’. The combined effect of body size and growth on incorporation rate may exacerbate this complication in large animals, such as ungulates and seals, and in the ‘slow’ tissues that are often used to study them (bone collagen, Koch 2007). In large animals the diet ingested during growth may give collagen an imprint that lasts for a long time after growth has ceased. Thus, the contribution of diets ingested after animals are fully grown may be difficult to detect. The confounding effects of growth on stable isotope analyses are probably a prevalent, and relatively unstudied, confounding factor in stable isotope field studies (Reich et al. 2008). © 2009 The Authors. Journal compilation © 2009 British Ecological Society, Functional Ecology, 23, 17–26 SIA in animal ecology 19 DIFFERENCES IN ISOTOPIC INCORPORATION AMONG TISSUES Tieszen et al. (1983) speculated that ‘more metabolically active tissues ... have faster turnover than less metabolically active tissues’. They supported this hypothesis with a negative correlation between oxygen consumption data measured in vitro and the half-life of 13C. Tieszen et al.’s (1983) statement has come to be interpreted to mean that both organisms and tissues with high metabolic rate, construed narrowly as a high rate of oxygen consumption, should have fast rates of isotopic incorporation (Hobson & Clark 1992; Voigt et al. 2003). Experimental evidence does not support this widely held assumption (Voigt et al. 2003; Carleton & Martínez del Rio 2005). Why do we find these discrepancies between experimental observations and a reasonable hypothesis? In their original article, Tieszen et al. (1983) melded two concepts: metabolic activity interpreted as the collection of anabolic and catabolic processes, and metabolic rate construed as the rate of oxygen consumption. MacAvoy et al.’s (2006) study exacerbated this conflation when they reported a negative correlation between mass-specific basal metabolic rate (MR/mb) and the half-life of isotopic incorporation. The half-life of a tissue-forming element (t1/2) scales with body mass to the 0·25 power (Carleton & Martínez del Rio 2005) and mass specific metabolic rate (MR/mb) scales with mb−1/4 (West et al. 1997). Therefore t1/2 must scale with (MR/mb)–1. MacAvoy et al.’s (2006) negative correlation is a consequence of the allometric dependence of both t1/2 and MR/mb on body mass. Although oxygen consumption is related to metabolism in the broad sense, the relationship is not direct and respiration rate can be uncoupled from some components of secondary metabolism (Marsh et al. 2001). Carleton & Martínez del Rio (2005) speculated that the primary determinant of the rate of isotopic incorporation in most tissues (whose isotopic composition is typically measured after lipids are extracted, Post 2007) is protein turnover (Lobley, 2003). This conjecture leads to two predictions: (i) the rate of isotopic incorporation into different organs/tissues should be ranked in the same order as their rate of protein turnover, and (ii) because physiologists have documented increases in protein synthesis resulting from increased protein intake in a variety of vertebrates (see Lobley 2003; Tsahar et al. 2007 and reviewed by Waterlow 2006), protein intake should influence isotopic incorporation rates. Testing Carleton & Martínez del Rio’s (2005) conjecture requires measuring protein turnover and isotopic incorporation concurrently. Although these measurements have not been done, there is experimental support for the hypotheses’ two predictions. Splanchnic organs with high rates of protein turnover such as the liver and intestine have higher rates of isotopic incorporation than collagen and muscle (Dalerum & Angerbjörn 2005, Fig. 1), and Tsahar et al. (2007) documented a 36–60% increase in the retention time of 15N in blood cells and plasma when they reduced the nitrogen content of the diet of a fruit-eating bird (Pycnonotus xanthopygos). Voigt et al. (2003) and Mirón Fig. 1. The time course of incorporation of the isotopic composition of a diet into an animal’s tissues can be described by either one- or two-compartment models. In house sparrows, Passer domesticus, whether a one- or a two-compartment model is best supported by data depends on tissue. Open bars in A represent tissues in which a one-compartment model is better supported by data, whereas closed bars represent those tissues in which data supported a twocompartment model. Panel B compares the fit of a one- and a twocompartment model to the incorporation of 13C into intestinal tissue of house sparrows after a change in diet. Note that the difference between the asymptotic value of δ13C and diet (i.e. Δ13Ctissue-diet) is smaller for the two compartment model. et al. (2006) reported contrasting rates of isotopic incorporation into the tissues of the same species of nectar feeding bat. These differences were explained by differences in protein intake (Mirón et al. 2006). Several studies have demonstrated increases in protein turnover with increased activity (Pikosky et al. 2006 and references therein). The link between protein turnover and isotopic incorporation rates suggests the intriguing possibility of the effect of activity on the rate of isotopic incorporation. To our knowledge, this possibility has not been yet tested. SHOULD WE USE MULTICOMPARTMENT MODELS? Physiologists studying protein turnover typically have relied on multi-compartment models (Waterlow 2006). Ayliffe et al. (2004), and Cerling et al. (2007a,b) have argued that by using-one compartment models, isotopic ecologists have over-simplified a complex process. Cerling et al. (2007a) proposed a graphical method they called the ‘reaction © 2009 The Authors. Journal compilation © 2009 British Ecological Society, Functional Ecology, 23, 17–26 20 N. Wolf et al. progress variable’ to diagnose whether isotopic incorporation data must be described by one- or multi-compartment models. Martínez del Rio & Anderson-Sprecher (2008) extended this method and proposed statistical estimates of average retention time for isotopes in multi-compartment systems and of the uncertainty associated with these estimates. They also proposed the use of model comparison approaches to assess the weight of evidence in favour of one- or multi-compartment models. We do not know yet if the application of these models will force us to reconsider the inferences hard won with studies that used one-compartment models. Because these patterns are strong, we suspect that they will be robust to model structure. However, details might change. For example, Carleton et al. (2008) found that using two compartment models consistently estimated a higher average retention time for carbon (Fig. 1) and a discrimination factor with a lower absolute magnitude. Mixing models MIXING MODELS Mixing models are the tool of choice to estimate the contribution of different sources to the tissues of an animal. In a mixing model we attempt to estimate the fractional contribution of an isotopic source to a tissue from the isotopic composition of the tissue and from the isotopic composition of the dietary sources. The simplest mixing model is of the form δT = fAδA + (1−fA)δB, where δ T is the isotopic composition of an animal’s tissue, δA and δB are the isotopic compositions of sources A and B, and fA and (1 − fA) are their relative fractional contributions (Phillips 2001). This model can be generalized to a linear system of N equations in N unknowns that allows estimating the contribution of N sources if one measures the composition of N – 1 isotopes in a tissue. Most studies rely on two isotopes (13C and 15N), and thus: δ13CT = fAδ13CA + fBδ13CB + fCδ13CC δ13CT = fAδ15NA + fBδ15NB + fCδ15NC 1 = fA + fB + fC eqn 3 We emphasize that fi estimates the contribution of source i to the isotopic composition of a tissue. It does not estimate the fraction of source i in the animal’s diet (see Martínez del Rio & Wolf 2005). Using eqn 3 to estimate contributions of different sources to diet assumes (i) that the elemental composition (i.e. the C : N ratio) of all the diets is equal, (ii) that the efficiency with which each element in each source is assimilated is the same, (iii) that there is no tissue to diet discrimination, and (iv) that there is no isotopic routing. The variation among sources in elemental ratios and in assimilation efficiency can be addressed relatively easily with concentrationdependent mixing models (Phillips & Koch 2002) and by adding an assimilation efficiency term to the models (Martínez del Rio & Wolf 2005). Available computer programs to estimate isotopic sources (Isosource, SIAR and SISUS, which you can easily find using Google) can address these complications. In eqn 3 the number of unknowns and equations is the same, and therefore one can easily find an analytical solution. Ecologists may face situations in which the number of sources (N) is higher than the minimal number of sources needed to constrain the system to a single solution (Phillips & Gregg 2003). In such a case, the number of possible solutions is infinite. Available computer programs can estimate the combinations of source proportions that satisfy eqn 3 and therefore provide researchers with a space of feasible solutions. TISSUE TO DIET DISCRIMINATION FACTORS The term ‘tissue to diet discrimination’ (denoted by Δ) refers to the difference in isotopic composition between a tissue and diet (i.e. Δ = δtissue−δdiet). If discrimination factors are measured experimentally, we can include them in a mixing model, δT = fA(δA + ΔA) + (1 − fA)(δB + ΔB). Discrimination factors vary among species, among tissues within a single species, and among diets (e.g. McCutchan et al. 2003), and are not often measured experimentally in field studies. Sometimes researchers used the average Δ value reported in large reviews. Because 3·4‰ is the average Δ15Ntissue−diet value reported in several reviews (Post 2002 and references therein), this number is frequently used as a discrimination factor, but other values are used as well. Some researchers use Δ values from related species fed on similar diets, but others used values from unrelated species fed on different diets (reviewed by Caut et al. 2008). How big an error do researchers make when they use the wrong discrimination factor? Assuming that ΔA and ΔB are equal (ΔA = ΔB = Δ), the difference between the estimated value ( fA*( Δ*)) and the real value ( fA) is given by: fA*( Δ*) − fA = Δ − Δ* , δ A − δB eqn 4 where Δ* is the guessed discrimination factor. Errors in the estimation of the fractional contribution of a source are smaller when the isotopic difference between the sources is large. Many studies rely on two isotopes to estimate the proportional contribution of three sources (see eqn 3). In such cases there are six possible unknown Δ values for three diets, greatly increasing the potential errors that result from using erroneous discrimination factors. Caut et al. (2008) found that the models worked best when they used discrimination factors estimated experimentally. When they used values from the literature, the estimated source proportions differed considerably from the real values. Ecologists interested in using mixing models are in a bind unless they conduct experiments (Haramis et al. 2001). When discrimination factors from the literature are used, a sensitivity analysis that examines the effect of variation in Δ is necessary. For the simplest mixing models with two sources and one isotope, the value of a source proportion depends only on two Δ values and the sensitivity analysis can be done by applying the following equation: fA = δ T − (δB + ΔB ) . (δ A + Δ A ) − (δB + ΔB ) eqn 5 The values of fA for the range of possible ΔA and ΔB values can be represented visually in a 3D plot with fA as the dependent variable. The tools to do a sensitivity analysis for the 2-isotope, 3-source case have not been developed. Even using Δ values measured experimentally is not without problems. Discrimination factors are measured with variation, and this variation will propagate when the mixing model is solved. Current methods do not account for variation in discrimination factors. Because mixing models are used frequently, finding out the effect of uncertainty in discrimination factors on the estimation of source proportions is an area in which theoretical progress is needed. In the future, studies that use discrimination factors in mixing models should be accompanied by discussion about how variation in their value, or errors in their estimation, contribute to uncertainty in the calculation of source proportions. © 2009 The Authors. Journal compilation © 2009 British Ecological Society, Functional Ecology, 23, 17–26 SIA in animal ecology 21 Routing Mixing models assume that assimilated nutrients are disassembled into their elemental components and that these elements are then reassembled into the molecules that make up tissues. This assumption is unrealistic. For example, the building blocks that animals use to manufacture tissues are not carbon atoms, but the carbon skeletons of a myriad of molecules. These carbon skeletons are conserved to various degrees. For example, amino acids can be indispensable and hence their carbon skeletons come from diet and are indispensable. The carbon skeletons of the dispensable amino acids come from either diet or are manufactured endogenously from other macromolecules (Bequette 2003). The differential allocation of isotopically distinct dietary components to different tissues is called ‘isotopic routing’ (Schwarcz 1991). Isotopic ecologists that work with omnivores that ingest diets in which carbohydrates (and sometimes lipids) are derived from one dietary source and protein is derived from another can face a quandary. They may find that using different tissues for isotopic analyses to reconstruct an animal’s diet might give different answers (Voigt et al. 2008). Worse, using a single type of tissue might give the wrong answer (Podlesak & McWilliams 2006). Although isotopic routing was reviewed 15 years ago by Ambrose & Norr (1993), the theme has received little attention from theoreticians and experimenters. Martínez del Rio & Wolf (2005) incorporated routing into a mixing model for δ13C by assuming that the carbon in protein was routed preferentially into tissue protein. Here we present a simplified graphical version of this model (Fig. 2). We assume that the animal ingests two dietary sources with contrasting carbon isotopic compositions, one that only contains protein and another one that contains only carbohydrates. We also assume that these diets provide other essential macronutrients, but that the amounts of these materials contribute little to the overall isotopic composition of diet. This simple model predicts that the isotopic composition of the animal’s tissue protein will be consistently higher than the value expected from a mixing model. The predictions of the model are consistent with the results of Ambrose & Norr (1993) and Podlesak & McWilliams (2006). Trophic discrimination factors USES OF ISOTOPIC DISCRIMINATION The observation that the isotopic composition of an animal’s tissues differs from that of their diet has been useful. DeNiro & Epstein (1981) noted that animal tissues were enriched in 15 N relative to their diets. This observation led to the conjecture that the content of 15N in animal tissues is biomagnified along the length of a food chain (Post 2002). This conjecture allows ecologists to use δ15N to estimate an animal’s trophic level (TL) using an equation devised by Vander Zanden et al. (1997) and modified by Post (2002): Fig. 2. A simple model of isotopic routing predicts that on diets in which sources with contrasting δ13C are comprised primarily of protein or carbohydrate, the δ13C of protein in tissues (solid curves) will be intermediate between that predicted by a mixing model (solid line) and that of the protein source (dashed parallel line). The model also predicts that the isotopic composition of lipid will be intermediate between that of a mixing line (dashed line, accounting for the −3‰ fractionation resulting from the synthesis of lipid from carbohydrate) and the δ13C of the carbohydrates source −3‰ (<39>Martinez del Rio & Wolf 2005). Closed points represent a diet with high protein quality (i.e. with amino acid composition that matches that of tissues), whereas open points represent a diet with low protein quality. 15 TL = λ + 15 δ N c − δ N base Δn eqn 6 where, δ15Nc is the nitrogen isotopic composition of the consumer, δ15Nbase is that of the food base, λ is the trophic level of the base (λ = 1 if the base is primary producers), and Δn is an estimate of the average increase in Δ15N per trophic level (Post 2002). We argue that using the wrong estimated discrimination can lead to large errors in the estimation of fractional source contributions. Eqn 6 relies on an estimated value of Δ15N, and yet it has been applied successfully many times. Vander Zanden & Rasmussen (2001) suggest that Δn is more variable for herbivores (primary consumers) than for carnivores. Therefore, using primary consumers (i.e. λ = 2) as a baseline reduces error in the estimation of TL. Vander Zanden et al. (1997) found a relatively tight positive correlation between the average trophic positions of freshwater fish estimated using δ15N and that estimated by gut content analyses. Although eqn 6 is frequently used in terrestrial systems, it has not been yet cross-validated. 15 N BIOACCUMULATION Bioaccumulation of toxicants along a food chain occurs because absorption is higher than elimination (Karasov & Martinez del Rio 2007). If the same explanation applies to 15 N, Δ15N should have a positive value if animals retain 15N preferentially over 14N (Martínez del Rio & Wolf 2005). Available evidence supports this observation. The materials excreted by the animals that have been measured tend to be © 2009 The Authors. Journal compilation © 2009 British Ecological Society, Functional Ecology, 23, 17–26 22 N. Wolf et al. isotopically lighter than tissues (reviewed by Tibbets et al. 2007). Sponheimer et al. (2003) questioned the 15N preferential excretion hypothesis. They measured δ15N in the food and excreta of llamas (Lama glama). They found that δ 15N values of excreta were not more negative than that of food. At steady state the isotopic composition of dietary inputs should equal that of outputs (Martínez del Rio & Wolf 2005). Therefore, finding that excreta are more depleted in 15N than diet is a sufficient condition for a positive Δ15N value, but it is not a necessary one. However, a positive Δ15N demands that excreta are depleted in 15N relative to the animal’s body – which was not measured by Sponheimer et al. (2003), and in all cases measured this seems to be the case (Tsahar et al. 2007). Olive et al. (2003) and Martínez del Rio & Wolf (2005) constructed isotopic mass balance models to explain a positive Δ15N. Martínez del Rio & Wolf’s (2005) model predicts that: (i) Δ15N values should decrease with increased protein quality in diet; (ii) Δ15N values should increase with diet’s protein content; (iii) Δ15N values should decrease with the efficiency of nitrogen deposition measured as the ratio between protein assimilation and protein loss; and (iv) Δ15N values should increase with fasting time. Prediction (i) is supported by Robbins et al. (2005) report of a highly significant interspecific negative correlation between Δ15N values and diet’s protein value. Prediction (ii) has mixed support: Pearson et al. (2003) found a positive linear relationship between Δ15Nbody-diet values and protein content in yellow-rumped warblers (Dendroica coronata) and Focken (2001) found an increase in Δ15Nbody-diet values with increased protein intake in Nile tilapia. In contrast, Tsahar et al. (2007) found lower Δ15N values in fruit-eating birds fed on diets with higher protein content, and Robbins et al. (2005) found no effect of protein content in their comparative study. To our knowledge, prediction (iii) has not been examined experimentally. Martínez del Rio & Wolf’s (2005), model predicts that Δ15N should increase with fasting time. This is a reasonable hypothesis that has been posed repeatedly (see Gannes et al. 1997) but that has received mixed support. Of eight studies on the effect of fasting on invertebrates, five found a significant enrichment in 15N and three found no effect. Because there are fewer fasting studies in vertebrates, the patterns are less clear. Hobson et al. (1993) found significant increases in δ15N values in fasting geese that lost c. 50% of their body mass. In fasting spawning salmon, only the liver became significantly enriched in 15N in post-spawning kelts relative to pre-spawning adults (Doucett et al. 1999). Castillo & Hatch (2007) fasted two species of lizards (Anolis carolinensis and Uta stansburiana) for 14 days and found that the tail muscles were not enriched in 15N relative to those of fed animals. However, they found that the δ15N values of excreta increased significantly from the beginning to the end of the fast. McCue (2008) also found that δ15N values in excreta increases along a 24-week fast in rattlesnakes (Crotalus atrox), without a change in body δ15N values. In fasting animals not all organs lose nitrogen to the same degree and in the same way (e.g. Doucett et al. 1999). Protein is lost in a tissue because protein is broken down by proteases into its component amino acids. The resulting amino acids are then de-aminated in situ, or exported to other organs (Caloin 2004). De- and trans-amination, should lead to 15 N-depleted nitrogenated by-products (ammonia, urea, and uric acid) and a remaining pool of enriched amino acids that can then be incorporated into proteins (Macko et al. 1986, 1987). Some organs such as muscle reduce their rate of protein synthesis during a fast (Waterlow 2006). Therefore, because these organs do not incorporate residual enriched amino acids, we should not expect them to become enriched. Other organs, such as liver retain high rates of protein synthesis during a fast (Waterlow 2006). They manufacture protein from the 15N-enriched pool of amino acids that remains from protein catabolism. The organs that will become 15N-enriched during a fast are those that maintain significant synthesis. DIFFERENCES IN TISSUE Δ13 C Tissue to diet discrimination factors differ among tissues (McCutchan et al. 2003). The variation among tissues is sometimes large. Reich et al. (2008) found that Δ13C varied from 0·9‰ to 2·62‰ in the tissues of loggerhead turtles (Caretta caretta). Other studies report differences of the same magnitude in Δ13C values among tissues (McCutchan et al. 2003). Lipid content and amino acid composition are two important candidates to explain inter-tissue differences in Δ13C values. Lipid synthesis is accompanied by depletion in 13C (DeNiro & Epstein 1977). Thus, some of the variation in Δ13C values is explained by a tissue’s lipid content (Post et al. 2007). Lipids are not the only factor that can cause differences in δ13C values among tissues. The δ13C values of amino acids of primary producers can range widely. O’Brien et al. (2005) reported differences of over 20‰ among the δ13C values of indispensable amino acids in the foliage of several plant species. They found that the δ13C values of the essential amino acids in larval food plants was an excellent predictor of the δ13C values of the essential amino acids in the eggs of nectarfeeding butterflies and moths. In contrast, because nonessentials were synthesized from carbon derived from adult food, their δ13C value was more homogeneous and reflected that of nectar sugars (see also Boggs 2009). Howland et al. (2003) found that the carbon isotopic composition of individual dispensable amino acids in pig (Sus scrofa) collagen was better predicted by the isotopic composition of bulk diet than by the composition of the individual amino acids in diet. Howland et al. (2003) found a tight correlation between the δ13C value of dietary indispensable amino acids and those in collagen for phenylalanine and leucine. The δ13C value of other dietary indispensable amino acids was a poor predictor of the δ13C value of those in collagen. This is a disturbing result with no adequate explanation. Howland et al. (2003) predicted accurately the isotopic composition of collagen from a mass balance model that includes the amino acid composition of collagen and the δ13C value of each individual amino acid in this tissue. © 2009 The Authors. Journal compilation © 2009 British Ecological Society, Functional Ecology, 23, 17–26 SIA in animal ecology 23 Fig 3. The range in δ15N values within a tissue (illustrated by horizontal bars at the bottom of the graph) appears to increase with trophic level. Some amino acids (‘source’ amino acids, sensu McClelland & Montoya 2002) appear to have consistently low δ15N values, whether others appear to be consistently 15N-enriched (‘trophic’ amino acids). The rotifer Brachionus plicatillis and the alga Tetraselmis suecica were cultured in the laboratory by McClelland & Montoya (2002). Popp et al. (2006) collected the data for the muscle of wild caught yellow-fin tuna (Thunnus albacares). Amino acids in bold are indispensable. DIFFERENCES IN TISSUE 15 Δ15 N TL = 1 + Tissues can differ greatly in δ15N value, and hence in Δ15Ntissue-diet. δ15N values varied among tissues from −0·64‰ to 1·65‰ among the tissues of loggerhead turtles (Reich et al. 2007). The difference in δ15N values among tissues can be explained by their amino acid content, and by the isotopic composition of individual amino acids. δ15N values vary among the amino acids of primary producers, and this variation seems to be amplified by the physiological processes of consumers (Fig. 6, McClelland & Montoya 2002; Popp et al. 2007). The δ15N value of amino acids in animal tissues seems to have a bimodal distribution (Fig. 3). Some amino acids appear to retain approximately the same nitrogen isotopic composition of food, whereas others become enriched in 15N by the animal’s metabolism. Popp et al. (2006) called the relatively 15N-enriched amino acids ‘trophic’, and the relatively 15 N-depleted ones, ‘source’. The heterogeneity in δ15N among amino acids within a tissue not only allows explaining variation in nitrogen isotopic composition among tissues, it also suggests that we might be able to estimate an animal’s trophic position from information contained within the animal’s tissues. McClelland & Montoya (2002) proposed using. Δ15Nglutamate–phenylalanine = δ15Nglutamate − δ15Nphenylalanine eqn 7 as an ‘internal’ index of trophic level. Recall that glutamate is a trophic, whereas phenylalanine is a source amino acid. McClelland & Montoya (2002) found that approximately Δ15Nglutamate–phenylalanine = 7‰. Therefore, Popp et al. (2006) suggested using the following modifications of eqn 12: 15 d N trophic − d N source 7 eqn 8 where d15Ntrophic is the average δ15N of the trophic amino acids and d15Nsource is the average δ15N of the source amino acids. Popp et al. (2006) compared the TL estimates using these equations with those obtained using eqn 6 and found roughly comparable results. The assumption that 7‰ represents a valid average increase in Δ15Nglutamate–phenylalanine or in Δ15Ntrophic–source per trophic level in all systems must be tested. It seems risky to derive a parameter that can be applied generally from a single study. Schmidt et al. (2004) measured the δ15N values in the amino acids of Antarctic krill (Euphausia superba). They found that females had more negative whole body bulk δ15N values than males. They also found that within each sex, the δ15N values of abdominal muscle were higher than that of the digestive gland. These differences in δ15N values were the result of differences in amino acid composition and in differences in isotopic composition among amino acids. They were also the result of inter-sex differences in δ15N values between the same amino acids. The trophic amino acids in females tended to be more depleted in 15N than those in males, especially in the digestive gland. Source amino acids differed less in δ15N values among tissues than trophic amino acids and did not differ between males and females. Schmidt et al. (2004) invoked similar physiological mechanism to those used in a previous section (15N bioaccumulation) to explain differences in δ15N values between the same amino acid among different individuals. Briefly, they speculated that trophic amino acids in individuals and tissues with high rates of transamination should be more enriched in 15N. The results of © 2009 The Authors. Journal compilation © 2009 British Ecological Society, Functional Ecology, 23, 17–26 24 N. Wolf et al. Schmidt et al.’s (2004) study holds two cautionary lessons. (i) If we apply eqn 8 to amino acids of the digestive gland, we infer that males have a much higher trophic level than females. If we apply it to data from the whole body, we infer that the difference in trophic level between males and females is much lower. The inferences that we make using TL equations can be tissue-dependent. (ii) Schmidt et al. (2004) relied on several lines of evidence to infer that male and female krill feed on the same trophic level. Thus, they concluded that differences in δ15N values between the trophic amino acids of males and females were the result of differences in amino acid metabolism. We need theory, more field observations, and more experiments to find out how much δ15N values vary among amino acids and to identify the factors that shape this variation. Routing, discrimination factors, and compound specific isotopic analyses Using isotopic analyses of bulk organic materials is an undoubtedly invaluable tool for ecologists. However, we have recognized that isotopic routing and the existence of discrimination must be understood when interpreting results of isotopic analyses of bulk materials. Compound specific analyses are an invaluable tool that, together with data on the composition of tissues can help interpret isotopic data. Indeed, it seems possible to classify different compounds depending on how sensitive they might be to routing and trophic enrichment. Consider amino acids. It appears that we can classify amino acids depending on the potential sources of their carbon and nitrogen (Table 1). Animals lack the ability to synthesize the amino acids that we consider indispensable, and hence the carbon in these must be derived from dietary sources without modification (reviewed by Karasov & Martinez del Rio 2007). In the situation depicted in Fig. 2, we can predict that the δ13C values in indispensable amino acids reflect directly those of the corresponding amino acids in diet, whereas the values of dispensable amino acids reflect a mixture of all sources. The reasons why the N in some amino acids is protected from isotopic enrichment are unknown, but Table 1. Amino acids can be classified as dispensable or indispensable depending on whether their carbon skeletons can be manufactured by the animal or not. They can also be classified as source or trophic, depending on whether their amino group is relatively enriched in 15N presumably due to frequent transamination events Indispensable Dispensable Source Trophic Phenylalanine Threonine Lysine Serine Glycine Tyrosine Isoleucine Leucine Valine Aspartic acid Glutamic acid Proline Alanine we speculate that they include how freely each amino acid exchanges nitrogen with others during trans-amination events. Whether the amino acids are dispensable or indispensable is not a potential criterion for 15N-enrichment (Fig. 3, Table 1). It appears that the dispensable and N-promiscuous amino acids involved in the transport and movement of nitrogen (alanine and glutamic acid) tend to be 15N-enriched, whereas those that are essential and not easily trans-aminated (phenylalanine and threonine) tend to be relatively 15N-depleted. The δ15N in some amino acids is perplexing. Proline and serine receive their nitrogen from glutamate (a 15N-enriched amino acid) during synthesis (Bequette, 2003). However, proline is highly 15N-enriched, whereas serine is 15N-depleted (Fig. 3). The mechanisms that lead to the source/trophic dichotomy among amino acids are fertile arena for the application of nutritional biochemistry to isotopic ecology. Other isotopes δ2H and δ18O values exhibit predictable patterns over the earth’s surface waters, and they have received enormous amounts of attention by researchers interested in finding the site of origin of animals in a field that can be properly called forensic ecology (reviewed by Rubenstein & Hobson 2004 and Bowen et al. 2005). However, few experimental studies inform the inferences of the huge data sets already generated by field observational research. For example, Doucett et al. (2007) found large differences (c. 100‰) in δ2H values between aquatic and terrestrial plants. Doucett et al. (2007) also measured the δ2H values in aquatic insects and fish and used mixing models to estimate the contribution of aquatic and terrestrial sources to the diets of these animals. They assumed that Δ2Htissues-diet = 0, and that the contribution of hydrogen (H) body water to the hydrogen bound in the organic compounds of tissues is negligible. These are two very risky assumptions. We know little about whether there is fractionation during the synthesis of biomolecules from precursors and body water, and very little about the relative contribution of hydrogen in body water and precursor dietary nutrients to the hydrogen bound to biomolecules. In another example, Birchall et al. (2005) reported large differences in δ2H values between the collagen of carnivores and herbivores, and assumed that these differences were the result of a trophic/biomagnification effect. This effect is plausible, but has so far, not been yet documented in a controlled feeding study. Estimating this putative biomagnification effect requires that diet and preformed water have the same δ2H value. In short, the many potential applications of D and 18O analyses in ecology demand that experimenters pay as much attention to them as they have to C and N. WE NEED MORE LABORATORY EXPERIMENTS AND MORE THEORY Perhaps not surprisingly, the number of observational field studies that apply stable isotopes to ecological problems far surpasses the number of experimental studies that aim to © 2009 The Authors. Journal compilation © 2009 British Ecological Society, Functional Ecology, 23, 17–26 SIA in animal ecology 25 clarify the mechanisms that explain the patterns that isotopic ecologists find. Ten years ago, Gannes et al. (1997, 1998) identified some of the areas that could be fruitfully explored by experimentally minded isotopic ecologists. Although stable isotopes have become firmly established as tools for animal ecologists, many questions about their use still remain, and most of these questions can only be resolved experimentally. We hope that this review has identified how much progress has been made in 10 years, but also how much remains to be done. Hence, we end it with a renewed call for experimentation. Because experiments and observations are most efficient at answering questions when informed by theory (National Research Council 2007), we add to our call an exhortation for the development of theoretical models. Acknowledgements The manuscript benefited from comments by Lenny Gannes, Kena Fox-Dobbs, and Seth Newsome. Research on stable isotopes in CMRs laboratory has been funded by NSF (IBN-0114016). This review is an updated and abbreviated form of material presented in Martínez del Rio et al. (2008). References Ambrose, S.H. & Norr, L. (1993) Carbon isotopic evidence for routing of dietary protein to bone collagen, and whole diet to bone apatite carbonate: Purified diet growth experiments. Molecular Archeology of Prehistoric Human Bone (eds J. Lambert & G. Grupe), pp. 11–22. Springer-Verlag, Berlin, Germany. Ayliffe, L.K., Cerling, T.E., Robinson, T., West, A.G., Sponheimer, M., Passey, B.H., Roeder, B., Dearing, M.D. & Ehleringer, J.R. (2004). Turnover of carbon isotopes in tail hair and breath CO2 of horses fed on an isotopically varied diet. Oecologia, 139, 11–22. Bartholomew, G.A. (1964) The roles of physiology and behaviour in the maintenance of homeostasis in the desert environment. Homeostasis and Feedback Mechanisms (ed. G.M. Hughes), pp. 7–29. Cambridge University Press, Cambridge. Bearhop, S., Waldron, S., Votier, S.C. & Furness, R.W. (2002) Factors that influence assimilation rates and fractionation of nitrogen and carbon stable isotopes in avian blood and feathers. Physiology and Biochemical Zoology, 75, 451– 458. Bequette, B.J. (2003) Amino acid metabolism in animals: An overview. Amino Acids in Animal Nutrition (ed. J.P.F. D’Mello), pp. 87–102. CABI Publishing, Wallingford. Birchall, J., O’Connell, T.C. Heaton, T.H.E. & Hedges, R.E.M. (2005), Hydrogen isotope ratios in animal body protein reflect trophic level. Journal of Animal Ecology, 74, 877–881. Boggs, C. (2009) Understanding insect life histories and senescence through a resource allocation lens. Functional Ecology, 23, 27–37. Bowen, G.J., Wassenaar, L.I. & Hobson, K.A. (2005) Global application of stable hydrogen and oxygen isotopes to wildlife forensics. Oecologia, 143, 337–348. Carleton, S.A., Kelly, L., Anderson-Sprecher, R. & Martinez del Rio, C. (2008) Should we use one or multi-compartment models to describe 13C incorporation into animal tissues? Rapid Comm in Mass Spectromotry, 22, 3008–3014. Caloin, M. (2004) Modeling of lipid and protein depletion during total starvation. American Journal of Physiology, 287, E790–E798. Carleton, S.A., & Martínez del Rio, C. (2005) The effect of cold-induced increased metabolic rate on the rate of 13C and 15N incorporation in house sparrows (Passer domesticus). Oecologia, 144, 226–232. Castillo, L.P. & Hatch, K.A. (2007) Fasting increases δ15N-values in the uric acid of Anolis carolinensis and Uta stansburiana as measured by nondestructive sampling. Rapid Communications in Mass Spectrometry, 21, 125–4128. Caut, S., Angulo, E. & Courchamp, F. (2008) Caution on isotopic model use for analyses of consumer diet. Canadian Journal of Zoology, 86: 438–445. Cerling, T.E., Bowen, G.J., Ehleringer, J.R. & Sponheimer M. (2007b) The reaction progress variable and isotope turnover in biological systems. Stable Isotopes as Indicators of Ecological Change (eds. T.E. Dawson & R.T.W. Siegwolf RTW), pp. 163–172. Academic Press, New York, NY. Cerling, T.E., Ayliffe, L.K., Dearing, M.D., Ehleringer, J.R., Passey, B.H., Podlesack, D.W., Torregrossa, A. & West, A.G. (2007a) Determining biological tissue turnover using stable isotopes: the reaction progress variable. Oecologia, 151, 175–189. Dalerum, F. & Angerbjörn, A. (2005) Resolving temporal variation in vertebrate diets using naturally occurring stable isotopes. Oecologia, 144, 647–658. DeNiro, M.J. & Epstein, S. (1977) Mechanism of carbon isotope fractionation associated with lipid synthesis. Science, 197, 261–263. DeNiro, M.J. & Epstein, S. (1981) Influence of diet on the distribution of nitrogen isotopes in animals. Geochimica et Cosmochimica Acta, 45, 341–351. Doucett, R.R., Booth, R.K., Power, G. & McKinley, V. (1999) Effects of the spawning migration on the nutritional status of anadromous Atlantic salmon (Salmo salar): insights from stable-isotope analysis. Canadian Journal of Fisheries and Aquatic Sciences, 56, 2172–2180. Doucett, R.R., Marks, J.C., Blinn, D.W., Caron, M. & Hungate, B.A. (2007) Measuring terrestrial subsidies to aquatic food webs using stable isotopes of hydrogen. Ecology, 88, 1587–1592. Focken, U. (2001) Stable isotopes in animal ecology: the effect of ration size on the trophic shift of C and N isotopes between feed and carcass. Isotopes in Environmental and Health Studies, 37, 199–211. Fry, B. & Arnold, C. (1982) Rapid 13C/12C turnover during growth of brown shrimp (Penaeus aztecus). Oecologia, 54, 200–20. Gannes, L.Z., Martinez del Rio, C. & Koch, P. (1998) Natural abundance variation in stable isotopes and their uses in animal physiological ecology. Comparative Biochemistry and Physiology, 119A, 725–737. Gannes, L.Z., O’Brien, D. & Martínez del Rio, C. (1997) Stable isotopes in animal ecology: assumptions, caveats, and a call for laboratory experiments. Ecology, 78, 1271–1276. Gillooly, J.F., Brown, J.H., West, G.B., Savage, V.M. & Charnov, E.L. (2001) Effects of Size and Temperature on Metabolic Rate. Science, 293, 2248– 2251. Haramis, G.M., Jorde, D.G., Macko, S.A., & Walker, J.L. (2001) Stable isotope analysis of canvasback winter diet in upper Chesapeake Bay. Auk, 118, 1008–1017. Hesslein, R.H., Hallard, K.A. & Ramal, P. (1993) Replacement of sulfur, carbon, and nitrogen in tissue of growing broad whitefish (Coregonus nasus) in response to a change in diet traced by δ34S, δ13C, and δ15N. Canadian Journal of Fisheries and Aquatic Sciences 50, 2071–2076. Hobson, K.A. & Clark, R.G. (1992) Assessing avian diets using stable isotopes I: Turnover of 13C in tissues. Condor, 94, 181–188. Hobson, K.A. & Wassenaar, L.I. (1999) Stable isotope ecology: an introduction. Oecologia, 120, 312–313. Hobson, K.A., Alsaukas, R.T. & Clark, R.G. (1993) Stable nitrogen isotope enrichment in avian tissues due to fasting and nutritional stress: Implications for isotopic analysis of diet. Condor 95, 388–394. Howland, M.R., Corr, L.T., Young, S.M.M., Jones, V., Jim, S., van der Merwe, N.J., Mitchell, A.D. & Evershed, R.P. (2003) Expression of the dietary isotope signal in the compound specific δ13C values of pig bone lipids and amino acids. International Journal of Osteoarchaeology, 13, 54–65. Jardine, T.D., McLatchy, D.L., Fairchild, W.L., Cujack, R.A. & Brown, S.B. (2004). Rapid carbon turnover during growth of Atlantic salmon (Salmo salar) in salt water, and evidence for reduced food consumption by growth stunts. Hydrobiologia, 527, 63–75. Karasov, W.H. & Martinez del Rio, C. (2007) Physiological Ecology: How Animals Process Energy, Nutrients, and Toxins. Princeton University Press, Princeton. Koch, P.L. (2007) Isotopic study of the biology of modern and fossil vertebrates. Stable Isotopes in Ecology and Environmental Science, 2nd edn (eds R. Michener & K. Lajtha), pp. 99–154. Blackwell Publishing, Boston. Lobley, G.E. (2003) Protein turnover – what does it mean for animal production. Canadian Journal of Animal Science, 83, 327–340. MacAvoy, S.E., Macko, S.A. & Arneson, L.S. (2005) Growth versus metabolic tissue replacement in mouse tissues determined by stable carbon and nitrogen isotope analysis. Canadian Journal of Zoology, 83, 631–641. MacAvoy, S.E., Macko, S.A. & Bassett, E. (2006) Correlation of metabolism with tissue carbon and nitrogen turnover rate in small mammals. Oecologia, 150, 190–201. Macko, S.A., Fogel Estep, M.L., Engel, M.H. & Hare, P.E. (1986) Kinetic fractionation of stable nitrogen isotopes during amino acid transamination. Geochimica et Cosmochimica Acta, 50, 2143–2153. Macko, S.A., Fogel Estep, M.L., Hare, P.E. & Hoering, T.C. (1987) Isotopic fractionation of nitrogen and carbon in the synthesis of amino acids by microorganisms. Chemical Geology, 65, 79–87. © 2009 The Authors. Journal compilation © 2009 British Ecological Society, Functional Ecology, 23, 17–26 26 N. Wolf et al. Marsh, A.G., Marxson, R.E. & Manahan. D.T. (2001) High macromolecular synthesis with low metabolic cost in Antractic sea urchin embryos. Science, 291, 1950–1952. Martínez del Rio, C. & Anderson-Sprecher R. (2008) Beyond the reactionprogress variable: the meaning and significance of isotopic incorporation data. Oecologia, 156, 765–772. Martínez del Rio, C. & Wolf, B.O. (2005) Mass balance models for animal isotopic ecology. Physiological Consequences of Feeding (eds M.A. Starck & T. Wang), pp. 141–174. Science Publishers, Enfield, New Hampshire. Martínez del Rio, C., Wolf, N., Carleton, S.A. & Gannes, L.Z. (in press) Isotopic ecology ten years after a call for more laboratory experiments. Biological Reviews (in press). McClelland, J.W. & Montoya, J.P. (2002) Trophic relationships and the nitrogen isotopic composition of amino acids in plankton. Ecology, 83, 2173–2180. McCue, M.D. (2008) Endogenous and Environmental Factors Influence the Dietary Fractionation of 13 C and 15 N in Hissing Cockroaches Gromphadorhina portentosa. Physiological and Biochemical Zoology, 81, 14–24. McCutchan, J.H. Jr, Lewis, W.M. Jr, Kendall, C. & McGrath, C.C. (2003) Variation in trophic shift for stable isotope ratios of carbon, nitrogen, and sulfur. Oikos, 102, 378–390. McIntyre, P.B. & Flecker, A.S. (2006) Rapid turnover of tissue nitrogen of primary consumers in tropical freshwaters. Oecologia, 148, 12–21. Mirón, L.L.M., Herrera, L.G.M., Ramírez, N.P. & Hobson, K.A. (2006). Effect of diet quality on carbon and nitrogen turnover and isotopic discrimination in blood of a New World nectarivorous bat. The Journal of Experimental Biology, 209, 541–548. National Research Council. (2007). The Role of Theory in Advancing 21-st Century Biology. The National Academies Press, Washington, DC. Newsome, S., Martínez del Rio, C., Phillips, D.L. & Bearhop, S. (2007) A niche for isotopic ecology. Frontiers in Ecology and the Environment, 5, 429– 436. O’Brien, D.M., Boggs, C.L. & Fogel, M.L. (2005) The amino acids used in reproduction by butterflies: a comparative study of dietary sources using compound specific stable isotope analysis. Physiology and Biochemical Zoology, 78, 819–827. Olive, P.J.W., Pinnegar, J.K., Polunin, N.V.C., Richards, G. & Welch, R. (2003) Isotope trophic-step fractionation: a dynamic equilibrium model. Journal of Animal Ecology 72, 608–617. Pearson, S.F., Levey, D.J., Greenberg, C.H. & Martínez del Rio, C. (2003) Effects of elemental composition on the incorporation of dietary nitrogen and carbon and isotopic signatures in an omnivorous songbird. Oecologia, 135, 516–523. Phillips, D.L. (2001) Mixing models in analyses of diet using multiple stable isotopes: a critique. Oecologia, 127, 166–170. Phillips, D.L. & Gregg, J.W. (2003) Source partitioning using stable isotopes: coping with too many sources. Oecologia, 136, 261–269. Phillips, D.L. & Koch, P.L. (2002) Incorporating concentration dependence in stable isotope mixing models. Oecologia, 130, 114–125. Phillips, D.L. & Eldridge, P.M. (2006) Estimating the timing of diet shifts using stable isotopes. Oecologia, 147, 195–203. Pikosky, M.A., Gaine, P.C., Martin, W.F., Grabarz, K.C., Ferrando, A.A., Wolfe, R.R. & Rodriguez, N.R. (2006) Aerobic exercise training increases skeletal muscle turnover in healthy adults at rest. The Journal of Nutrition, 136: 379–383. Podlesak, D.W. & McWilliams, S.R. (2006) Metabolic routing of dietary nutrients in birds: effects of diet quality and macronutrient composition revealed using stable isotopes. Physiology and Biochemical Zoology, 70, 534–549. Popp, B.N., Graham, B.S., Olson, R.J., Hannides, C.C.S., Lott, M., Lopez-Ibarra, G. & Galvan-Magana, F. (2007) Insight into the trophic ecology of yellowfin tuna, Thunnus albacares, from compound-specific nitrogen isotope analysis of proteinaceous amino acids. Isotopes as Tracers in Ecological Change (eds T. Dawson & R. Seigwolf), pp. 173–190. Elsevier, New York. Post, D.M. (2002) Using stable isotopes to estimate trophic position: models, methods, and assumptions. Ecology, 83, 703–718. Post, D.M., Layman, C.A., Arrington, D.A., Takimoto, G., Quattrochi, J. & Montaña, C.G. (2007). Getting to the fat of the matter: models, methods, and assumptions for dealing with lipids in stable isotope analysis. Oecologia, 152, 179–189. Reich, K.J., Bjorndal, K.A. & Martínez del Rio C. (2008) Effects of growth and tissue type on the kinetics of 13C and 15N incorporation in a rapidly growing ectotherm. Oecologia, 155, 651–658. Robbins, C.T., Felicetti, L.A. & Sponheimer, M. (2005) The effects dietary protein quality on nitrogen isotope discrimination in mammals and birds. Oecologia, 144, 534–540. Rubenstein, D.R. & Hobson, K.A. (2004) From birds to butterflies: animal movement patterns and stable isotopes. Trends in Ecology and Evolution, 19, 256–263. Schmidt, K., McClelland, J.W., Mente, E., Montoya, J.P., Atkinson, A. & Voss, M. (2004) Trophic-level interpretation based on delta N-15 values: Implications of tissue-specific fractionation and amino acid composition. Marine Ecology Progress Series, 266, 43–58. Schwarcz, H.P. (1991) Some theoretical aspects of isotope paleodiet studies. Journal of Archaeological Science, 18, 261–275. Sponheimer, M., Robinson, T., Roeder, B., Ayliffe, L., Passey, B., Cerling, T., Dearing, D. & Ehleringer, J. (2003). An Experimental Study of Nitrogen Flux in Llamas: Is 14N Preferentially Excreted? Journal of Archaeological Science, 30, 1649–1655. Suzuki, K.W., Kasai, A., Nakayama, K. & Tanaka, M. (2005) Differential isotopic enrichment and half life among tissues in Japanese temperate bass (Lateolabrax japonicus) juveniles: implications for analyzing migration. Canadian Journal of Fisheries and Aquatic Sciences, 62, 671–678. Tibbets, T., Wheeless, L.A. & Martínez del Rio, C. (2007) Isotopic enrichment without change in diet: an ontogenetic shift in δ15N during insect metamorphosis. Functional Ecology, 22, 109–113. Tieszen, L.L., Boutton, T.W., Tesdahl, K.G. & Slade, N.A. (1983) Fractionation and turnover of stable carbon isotopes in animal tissues: implications for δ13C analysis of diet. Oecologia, 57, 32–37. Tsahar, E., Wolf, N., Ishaki, I., Arad, Z. & Martínez del Rio, C. (2007) Diet influences the rate of 15N incorporation in blood cells and plasma of yellow-vented bulbuls (Pycnonotus xanthopygos). The Journal of Experimental Biology, 211, 459–465. Vander Zanden, M.J. & Rasmussen, J.B. (2001) Variation in d15N and d13C trophic fractionation: Implications for aquatic food web studies. Limnology and Oceanography, 46, 2061–2066. Vander Zanden, M.J., Cabana, G. & Rasmussen, J.B. (1997) Comparing trophic position of freshwater littoral fish species using stable nitrogen isotopes (δ15N) and literature dietary data. Canadian Journal of Fisheries and Aquatic Sciences, 54, 1142–1158. Voigt, C.C., Matt, F., Michener, R. & Kunz, T.H. (2003). Low turnover rates of carbon isotopes in tissues of two nectar-feeding bat species. The Journal of Experimental Biology, 206, 1419–1427. Voigt, C., Katja, R., Michener, R. & Speakman, J. (2008) Nutrient routing in omnivorous animals tracked by stable carbon isotopes in tissue and exhaled breath. Oecologia 157, 31–40. Waterlow, J.C. (2006) Protein Turnover. CABI Publishing, Wallingford. West, G.B., Brown, J.H. & Enquist, B.J. (1997) A general model for the origin of allometric scaling laws in biology. Science, 276, 122–126. Witting, D.A., Chambers, R.C., Bosley, K.L. & Wainright, S.C. (2004) Experimental evaluation of ontogenetic diet transitions in summer flounder (Paralichtys dentatus), using stable isotopes as diet tracers. Canadian Journal of Fisheries and Aquatic Sciences, 61, 2069–2084. Received 16 September 2008; accepted 19 November 2008 Handling Editor: David Raubenheimer © 2009 The Authors. Journal compilation © 2009 British Ecological Society, Functional Ecology, 23, 17–26