Outer Layers (Thermosphere Ionosphere)
advertisement

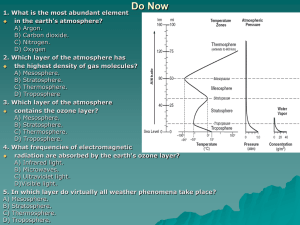
Outer Layers (Thermosphere Ionosphere): Energy Input to the Upper Atmosphere: • We saw earlier that the energy supplied to the Troposphere comes from heat radiating from the ground. • Above the troposphere the sources of energy are different and less uniform. • For the most part the source is always the same. LIGHT • Different parts of the Sun’s ‘spectrum’ of emitted light are absorbed at different regions of the atmosphere. Atmospheric Layers and Solar Input: Summary Energy Deposition: We’ve already covered how the surface of the Earth absorbs sunlight and warms to provide the source of heat in the troposphere. How is energy absorbed into the atmosphere? • Photochemistry: Sunlight breaks apart a molecule. OH + light ⇒ O + H • Photoionization: Sunlight breaks a neutral molecule or atom in to charged particles. O2 + light ⇒ O2+ + e- So How Does This Heat the Atmosphere? The amount of energy it takes to break apart an atom or molecule (or to ionize it) is a fixed amount, a threshold. The amount of energy in light depends on frequency and wavelength (color). All light with a shorter wavelength (bluer) or a higher frequency (bluer again!) than the light with the threshold energy can trigger the breakup. It is the products of the breakup that carry any extra energy away. XV + light ⇒ YV1 + ZV2 From this perspective we are thinking of temperature and velocity (V, V1, and V2) being equivalent, which they are! (note that wind is something differente from what we are discussing here! ). Where is the energy deposited? Incoming energy is absorbed only by those atoms and molecules that it can break apart. Each allowed interaction has a chance of happening when a photon of sufficient energy passes by. Convention has us call this chance a ‘cross-section’, which is equivalent to treating each atom or molecule as a billiard ball. Using the cross-section, the chance of an interaction is equal to the ‘size’ of the atom or molecule. A typical cross-section is 10-18 cm or one interaction for every 1000000000000000000 photons that pass by an atom!!!! Many Targets Fill the Hole: As the number of particles in a given area increases, the chances of an interaction do as well. Eventually there may be enough particles that they ‘block’ the light. We call the amount of blocking the ‘optical depth’ of the target. If most light passes through ‘column’ of the atmosphere, it is referred to as ‘optically thin’. If most is blocked, then it is ‘optically thick’ Hitting the Wall: If the atmosphere was completely uniform in density, then energy would be deposited over a wide range of altitudes. However, the atmosphere is not uniform, but becomes less dense as we go higher. This means that there is much more of a target below the level where the atmosphere becomes optically thick than above. In cases like this, the rate of the reaction becomes sharply peaked on the point where optical depth approaches 100%, with little happening above and almost nothing below. Energy Deposition Regions: In the atmosphere then, this means that the rate and location of energy input depend on: • The density of the target. • The energy of the reaction. • The composition of the atmosphere. • The solar spectrum. Thus, the rate and altitude of energy input to the upper atmosphere is stratified into regions that are unique to the conditions on the Earth. Escape of Atmospheric Energy: If the absorption of photons increases the temperature of the atmosphere, then the escape of photons should cool it. How does this happen? Greenhouse Gasses Solar 4) 4) 3) 3) 2) 2) 1) 1) Earth Energy Deposition/Loss and the Structure of the Upper Atmosphere: Above the troposphere, many of the thermal properties of the upper atmosphere are determined by where and how much energy is deposited. It is this process that controls the extent of the atmosphere layers. The Stratosphere: The stratosphere is the atmospheric layer directly above the tropopause. It is the first region characterized by atmospheric energy deposition. The primary source of energy in the stratosphere is photochemical. Incoming ultraviolet light is absorbed first by O2 and then by Ozone (O3). Much of the O3 that is destroyed is at the top of the stratosphere. The Role of Ozone: Remember from our earlier lecture the effect of Chlorofluorocarbons on Ozone. Cl + O3 ⇒ ClO + O2 ClO + O ⇒ Cl + O2 The above is called a catalytic reaction. The O3 is destroyed, but not Cl! Energy isn’t delivered in this cycle, BUT… Without O3 to absorb UV radiation the heating balance of the stratosphere and is affected! The Mesosphere: Above the Stratopause is another, thin atmospheric layer called the Mesosphere or “Middle Sphere”. Energy input to the Mesosphere comes from the formation of Ozone, which occurs near the stratopause (the bottom). • Like the troposphere, temps in the mesosphere drop rapidly with altitude. And we now understand that this is due to cooling at the top and heating at the bottom! • The cooling of the mesophere is due mainly to CO2. -100 32 Temperature (F) • The rapid drop in temperature makes this the most turbulent part of the atmosphere. The Thermosphere & Ionosphere Above the Mesopause the atmosphere begins to heat rapidly as the atmosphere absorbs solar UV and X-Ray radiation. The thermosphere is the hottest and thinnest region of the atmosphere (effectively space). The thermosphere is characterized by four features. • Mixing of atmospheric gasses begins to break down. • It derives much of its energy from photoionization. • The charged atmosphere connects directly to the near space environment. -100 1500 (750) Temperature (F) • It is the most thermally variable. Shaken, Not Stirred: The atmosphere consists of several gasses that are all mixed together, including O2, N2, CO2, Ar, He, CH4, etc. The altitude distribution of the atmosphere (depends on average mass, temperature, and, gravity). In the Troposphere and Mesosphere convection keeps these gasses well mixed. In the Stratosphere and Thermosphere convection is less important. Two processes affect how the atmosphere is mixed. • Eddy Mixing: Eddy mixing is the process of using small random motions in a gas to mix constituents of different masses. • Molecular Diffusion: Diffusive mixing is the process of “settling out”, where each gas behaves as though it were alone, with its own altitude distribution. Transitions: At each level in the atmosphere there is ”characteristic time scale” over which eddy mixing or diffusive mixing occur. Which ever occurs faster determines the structure of the atmosphere. • In the Stratosphere eddy mixing is much more efficient at organizing the atmosphere than diffusion. • In the Thermosphere eddy mixing is less effective. Above ~110 km, the pace of diffusion begins to dominate. • The altitude where this occurs is called the “Turbopause” (end of turbulence). Thermosphere Variability: Because the thermosphere is so thin, small changes in energy input have a large effect on temperature. • Day time temperatures can reach 1500C. • At night temperatures drop as much as 1000C. • Heating of the Thermosphere comes form the most variable part of the Sun’s energy output. • When the Sun is “Active” the Thermosphere gets hotter and extends out into space farther. This can affect the orbits of satellites. • High activity periods occur predictably every 11 years. This is called the Solar Cycle. The Ionosphere: The “Ionosphere” isn’t really an atmospheric region, but rather a series of layers where photoionization rates are greatest. The ionosphere is a manifestation of the optical depth effects described earlier. Different reactions with sunlight set up different regions. While ions are produced throughout the atmosphere, there are three maximum areas. • The D-Region • The E-Region • The F-Region We can “see” the ionosphere layers by reflecting radio waves off them. Solar Input: The ionosphere is generated by different parts of the solar spectrum. The ion populations are different in each region. Ion Populations: • The D region is located in the Mesosphere from 60-90 km. • Its ion population is dominated by NO+ and O2+ • The E region is located in the Thermosphere from 90-150 km. • Its ion population is also dominated by NO+ and O2+ • The F region is located in the Thermosphere from 150-800 km. • Its ion population is dominated by O+