Documento descargado de http://www.elsevier.es el 01/10/2016. Copia para uso personal, se prohíbe la transmisión de este documento por cualquier medio o formato.
Rev Esp Med Nucl Imagen Mol. 2012;31(3):117–123
Original article
Sentinel lymph node biopsy in patients with operable breast cancer treated with
neoadjuvant chemotherapy夽
Á.C. Rebollo-Aguirre a,∗ , M. Gallego-Peinado b , S. Menjón-Beltrán c , J. García-García c , E. Pastor-Pons d ,
C.E. Chamorro-Santos e , C. Ramos-Font f , A. Salamanca-Ballesteros c , J.M. Llamas-Elvira a ,
N. Olea-Serrano g
a
Servicio de Medicina Nuclear, Hospital Universitario Virgen de las Nieves, Granada, Spain
Servicio de Medicina Nuclear, Hospital Universitario Santa Lucía, Cartagena, Murcia, Spain
Servicio de Ginecología, Hospital Universitario Virgen de las Nieves, Granada, Spain
d
Servicio de Radiodiagnóstico, Hospital Universitario Virgen de las Nieves, Granada, Spain
e
Servicio de Anatomía Patológica, Hospital Universitario Virgen de las Nieves, Granada, Spain
f
Servicio de Medicina Nuclear, Hospital Juan Ramón Jiménez, Huelva, Spain
g
Departamento de Radiología y Medicina Física, Facultad de Medicina, Universidad de Granada, Spain
b
c
a r t i c l e
i n f o
Article history:
Received 29 March 2011
Accepted 26 April 2011
Keywords:
Breast cancer
Sentinel lymph node
Neoadjuvant chemotherapy
Primary systemic therapy
a b s t r a c t
Aim: To evaluate the accuracy of sentinel lymph node biopsy (SLNB) in operable breast cancer patients
treated with neoadjuvant chemotherapy (NAC).
Material and methods: Between January 2008 and 2011, 88 women, mean age 49.4 years, with infiltrating breast carcinoma, were studied prospectively. Patients were T1–3, N0–1, M0. Prior to surgery,
the patients received chemotherapy (epirubicin/cyclophosphamide, docetaxel), and trastuzumab in
Her2/neu-positive patients. Axillary status was established by physical examination, ultrasound-guided
core needle biopsy of any suspicious lymph node. The day before surgery, 74–111 MBq of 99m Tc-albumin
nanocolloid was injected periareolarly. All patients underwent breast surgery, with SLNB, followed by
complete axillary lymph node dissection (ALND). Sentinel lymph node (SLN) was examined by frozen
sections, hematoxylin–eosin staining and immunohistochemical analysis or One Step Nucleic Acid Amplification (OSNA).
Results: Mean tumor size: 3.5 cm, histologic type: 69 invasive ductal, 16 invasive lobular and 3 others.
Thirty-seven patients had clinical/ultrasound node-positive at presentation. Clinical response of primary
tumor to NAC: complete in 38, partial in 45, and stable disease in 5 patients. A pathological complete
response was achieved in 25. All patients were clinically node-negative after NAC.
SLN identification rate was 92.0%. Six of 7 patients in whom SLN was not found had clinical/ultrasound
positive axilla before NAC. SLN accurately determined the axillary status in 96.5%. False negative rate was
8.3%. In 69.4% of patients, SLN was the only positive node. The mean number of SLN removed was 1.7 and
nodes resected from the ALND were 13.2.
Conclusion: SLN biopsy after NAC can predict the axillary status with a high accuracy in patients with
breast cancer, avoiding unnecessary ALND.
© 2011 Elsevier España, S.L. and SEMNIM. All rights reserved.
Biopsia del ganglio centinela en pacientes con cáncer de mama operable
tratadas con quimioterapia neoadyuvante
r e s u m e n
Palabras clave:
Cáncer de mama
Ganglio centinela
Quimioterapia neoadyuvante
Terapia sistémica primaria
Objetivo: Validar la biopsia selectiva del ganglio centinela (BGC) en pacientes con cáncer de mama tratadas
con quimioterapia neoadyuvante.
Material y métodos: Estudio prospectivo de enero de 2008 a enero de 2011, 88 pacientes con una
edad media de 49,4 años, con cáncer de mama infiltrante T1-3, N0-1, M0, tratadas con epirrubicina/ciclofosfamida, docetaxel y trastuzumab en Her2/neu positivas. El estatus axilar se estableció por
exploración física, ecografía axilar y punción ecoguiada de ganglios sospechosos. El día antes de la
cirugía se inyectaron periareolarmente 74-111 MBq de 99m Tc-nanocoloide de albúmina. En todas se realizó cirugía mamaria, BGC y linfadenectomía axilar. El ganglio centinela (GC) se analizó por cortes de
congelación, hematoxilina-eosina, inmunohistoquímica u OSNA.
Resultados: El tamaño medio del tumor fue de 3,5 cm. Según el tipo histológico, 69 se clasificaron como
ductal infiltrante, 16 como lobulillar infiltrante y 3 como de otro tipo. Treinta y siete pacientes tenían
axila clínica/ecográfica positiva al diagnóstico. La respuesta clínica del tumor primario fue: 38 completa,
夽 Please cite this article as: Rebollo-Aguirre ÁC, et al. Biopsia del ganglio centinela en pacientes con cáncer de mama operable tratadas con quimioterapia neoadyuvante.
Rev Esp Med Nucl Imagen Mol. 2012;31:117–23.
∗ Corresponding author.
E-mail address: angelc.rebollo.sspa@juntadeandalucia.es (Á.C. Rebollo-Aguirre).
2253-8089/$ – see front matter © 2011 Elsevier España, S.L. and SEMNIM. All rights reserved.
Documento descargado de http://www.elsevier.es el 01/10/2016. Copia para uso personal, se prohíbe la transmisión de este documento por cualquier medio o formato.
118
Á.C. Rebollo-Aguirre et al. / Rev Esp Med Nucl Imagen Mol. 2012;31(3):117–123
45 parcial, 5 no respuesta. En todas las pacientes la axila fue clínica/ecográfica negativa después del
tratamiento. En 25 casos hubo respuesta patológica completa en el tumor primario.
El porcentaje de identificación del GC fue del 92,0%, 6 de las 7 pacientes sin migración eran axila
clínica/ecográfica positiva al diagnóstico. En el 96,3% de los casos el GC determinó correctamente el estatus axilar. La tasa de falsos negativos fue del 8,3%. En el 69,4% de los casos el GC era el único afectado de
la axila. El número medio de GC identificados fue 1,7 y el de ganglios axilares extirpados fue 13,2.
Conclusión: La BGC es una técnica factible en pacientes con cáncer de mama tratadas con quimioterapia
neoadyuvante, pudiendo evitar linfadenectomías innecesarias.
© 2011 Elsevier España, S.L. y SEMNIM. Todos los derechos reservados.
Introduction
Breast cancer is the most frequent tumor in Western women,
with the probability of developing the disease before the age of
75 years having been estimated at 8%. This incidence is increasing at an annual rhythm of 1–2% due to the progressive ageing
of the population. However, a continued reduction has been
observed in the mortality by this disease and 76% of Spanish
women with breast cancer remain alive 5 years after diagnosis.
This reduction in mortality is due to two fundamental pillars:
early diagnosis and the advances in surgical and oncological
treatment.1
There are two fundamental premises in breast cancer surgery:
acceptation of conservative surgery as the standard treatment and
the doubts related to the therapeutic efficacy of axillary lymphadenectomy. In addition, lymphadenectomy produces marked
morbidity, with severe and definitive complications such as
lymphedema. At present increasingly more breast cancers are
diagnosed in early stages, questioning the role of the lymphadenectomy, since most of these patients do not have axillary
involvement.2
Scintigraphic and intraoperative detection of the sentinel lymph
node (SLN) is currently considered as a standard technique in the
surgical treatment of breast cancer with the aim of avoiding unnecessary axillary lymph node dissection at the time of disease staging
thereby decreasing the morbidity of this procedure and allowing a
more extensive histopathological study. In the last years there has
been a clear trend to an increase in the number of patients who may
benefit from SLNB in breast cancer, including different indications
from those initially considered.3–6
Neoadjuvant chemotherapy (NCT) (preoperative, induction, primary systemic therapy) has been the standard treatment in women
with inoperable, locally advanced or inflammatory, breast cancer,
with evidence supporting its use in early stages.7,8 The indication
of NCT is adequate in patients with tumors of 3 cm or even smaller
when conservative surgery is not possible. Patients with operable breast cancer have tumors in stages I to III-A (T1–T3, N0–N1,
M0) and may be treated with multiple therapeutic strategies.7 The
administration of NCT has the following possible advantages: it
converts an initially non-surgical breast cancer into one that is
operable, increases the number of conservative surgeries, evaluates the sensitivity of the tumor to chemotherapy in vivo, initiates
systemic treatment early and is a model for clinical or translational
investigation. The potential disadvantages include: delay in local
treatment, the risk of progression during treatment and imprecise
axillary staging.8
Neoadjuvant chemotherapy has classically been considered a
contraindication for SLNB since the fibrotic changes produced in the
primary tumor and in the axillary region (in both the lymph nodes
and the lymph channels) and the presence of cellular material or
metastatic emboli in the lymph channels of patients with tumors
in more advanced stages could cause obstruction of lymph flow or
deviation to other lymph node stations. Likewise, the response of
the lymph nodes to chemotherapy may not be uniform, not being
the sentinel lymph node (SLN) in these cases and reflecting axillary
status. Nonetheless, numerous groups have reported their experience with SLNB after NCT, albeit with contradictory results.9–12
At present the convenience of performing SLNB prior to or after
primary systemic treatment is under question, as are the advantages or disadvantages of when it should be performed. If the
fundamental objectives of SLNB are correct staging and avoidance
of unnecessary lymphadenectomies in patients without lymph
node involvement, it seems logical that both objectives may be
achieved if this diagnostic procedure is selectively carried out prior
to or after NCT.12–15
The main objective of this study was to attempt to validate
SLNB with periareolar injection of a radiotracer in patients with
operable breast cancer previously treated with primary systemic
chemotherapy.
Materials and methods
Study design
We performed a descriptive study of a series of prospective
cases. The method followed for accreditation of the procedure
is statistical validation of a consecutive registry of cases. The
relationship between the histological results of the SLN and its correspondence with axillary lymph node status was analyzed as were
the main clinical characteristics of the patients: age, menopausal
status, size and localization of the tumor in the breast, axillary status at diagnosis, histological tumor type, clinical and pathological
response of the tumor. We also compared the results obtained with
our values in the SLNB validation phase in patients with breast
cancer in early stages without primary systemic treatment.16
Patients
From January 2008 to January 2011 we prospectively studied
93 women consecutively selected from among patients referred
to the Functional Oncologic Breast Cancer Unit of the University
Hospital Virgen de las Nieves in Granada who fulfilled the following
inclusion criteria: operable breast cancer histologically confirmed
by thick needle biopsy puncture who had undergone preoperative
primary systemic chemotherapy, breast cancer surgery and SLNB
with immediate axillary lymphadenectomy. We excluded women
with inflammatory breast carcinoma, previous breast surgery or
axillary or breast radiotherapy, multifocal or multicentric tumors,
systemic metastatic disease or second neoplasm, women who were
pregnant or in lactation, under 18 years of age, with a history of
allergy to human albumin or who withdrew consent at any time
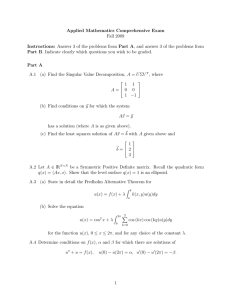
during the study. A total of 88 patients (Fig. 1) were finally included.
Axillary status was established by physical examination, axillary
ultrasonography and ultrasound-guided puncture of the suspicious
lymph nodes at both diagnosis and at the end of chemotherapy.
Evaluation of the grade of clinical response to NCT was made from
the changes produced in tumor size by physical examination and
conventional imaging techniques, being classified as: complete
response (cCR), partial response (cPR) or no response (cNR).
Documento descargado de http://www.elsevier.es el 01/10/2016. Copia para uso personal, se prohíbe la transmisión de este documento por cualquier medio o formato.
Á.C. Rebollo-Aguirre et al. / Rev Esp Med Nucl Imagen Mol. 2012;31(3):117–123
Patients fulfilling the
inclusion criteria
(n = 93).
Withdrawal of consent for
transfer to another hospital center
(n = 3).
Patients completing
neoadjuvant chemotherapy
(n = 90).
Patients without complete surgery:
‐ Refusal of surgery
‐ Anesthesia complications
(n = 2)
Patients evaluated
(n = 88)
Fig. 1. CONSORT flow diagram of the patients.
The patients received the following sequential chemotherapy
regimen: 4 cycles of epirubicin (90 mg/m2 ) and cyclophosphamide
(600 mg/m2 ) every 21 days, followed by 4 cycles of docetaxel
(100 mg/m2 ) combined with trastuzumab (6 mg/kg, 8 mg/kg day
1) in patients with overexpression of HER-2 every 21 days.
Written informed consent was obtained from all the patients.
The study was approved by the Ethical Committee of Clinical Trials
of our hospital.
Scintigraphic and intraoperative detection of the sentinel lymph
node
119
(ITC, size less than 0.2 mm), micrometastasis (from 0.2 to 2 mm)
or macrometastasis (size greater than 2 mm).
In the remaining 49 patients the OSNA (one-step amplification of nucleic acid) molecular procedure was applied. The results
were expressed semi-quantitatively in three different categories
according to the correlation between the number of tumoral cells
per volume of tissue and the number of mRNA copies of cytokeratin 19 (CK19) per tumoral cell: macrometastasis (more than
5000 copies/l), micrometastasis (from 250 and 5000 copies/l) or
absence of metastasis (less than 250 copies/l).
The lymph nodes of the lymphadenectomy were analyzed with
H–E staining and immunohistochemistry when considered necessary. Pathological response of the primary tumor to NCT was
evaluated in the breast piece, being classified as: complete pathological response (pCR) or persistence of infiltrating residual disease
(pNR).
Statistical analysis
In the descriptive analysis the quantitative variables were
expressed as mean, typical deviation, minimum and maximum and
for the description of the qualitative variables we calculated the
frequency and relative percentage in the population. Contingency
tables were designed (table 2 × 2) to calculate the sensitivity, specificity, positive predictive value (PPV), negative predictive value
(NPV) and the diagnostic precision of the technique, accompanied
by estimation of the confidence interval of 95% (CI).
For the analysis of the differences in the categoric variables
we used the Pearson Chi-square test, the Fisher exact test with a
bilateral perspective or the Mann–Whitney U-test and for the differences in the mean values of the continuous values we used the
Student’s t-test. A P value < 0.05 was considered as statistically significant. Data were analyzed with the EpiDat version 3.1 and SPSS
version 13.0.
Results
The afternoon prior to surgery, 99mTc-albumin nanocolloid
(Nanocoll® ) was administered by four intradermic/subdermic
injections at the positions of 3, 6, 9 and 12 o’clock around the areola
of the affected breast. The volume of each injection was 0.2–0.3 ml
with a total activity administered ranging from 74 to 111 MBq
(2–3 mCi). Immediately after injection of the radiotracer a scintigraphy was carried out with planar acquisition of static images at
least in two projections (anterior and lateral or anterior oblique
at 45◦ ) until visualization of the SLN, with the following technical
parameters of acquisition: low energy and general purpose collimator, energy window: 140 ± 10% keV, matrix 256 × 256, 180 s per
image. The SLN was considered the lymph node(s) visualized, especially if connected to a lymphatic channel. Once the SLN has been
identified its location is marked with a waterproof ink felt pen on
the skin of the patient who is placed in a position similar to that of
the surgical intervention. At the time of surgery, the SLN was considered the lymph node identified in the territory determined by
the lymphoscintigraphy presenting the greatest activity with the
gamma probe in the surgical bed and those surpassing 10% of the
activity of the lymph node with greatest uptake (rule of 10%).
Anatomopathologic study
The SLN was analyzed by two techniques throughout this study.
In the first 32 patients intraoperative histological analysis of the
SLN was performed with slices by freezing and deferred analysis
with hematoxylin–eosin (H–E) staining and immunohistochemistry with anti-cytokeratin antibodies (clone AE1/AE3). The SLN
was classified as: negative for metastasis, isolated tumoral cells
The main clinical and pathological characteristics at diagnosis of
the patients included in the study are summarized in Table 1. Anatomopathological confirmation was obtained in 24 of the 37 patients
clinically and/or ultrasonographically axilla-positive at diagnosis.
Of the remaining 13 women, the biopsy was negative in 3 and
could not be performed in 10 due to technical difficulties related
to the anatomical localization of the suspicious lymph node or its
proximity to vascular structures.
Following the NCT 38 patients (43.2%) presented cCR at the level
of the primary tumor, 45 patients (51.1%) had cPR and 5 patients
(5.7%) showed cNR. All the patients were clinically and echographically axilla-negative after treatment.
Surgery was performed from 17 to 23 h after the administration
of the radiotracer, consisting in conservative breast surgery in 82
of the women studied (93.2%) and modified radical mastectomy in
6 (6.8%). Lymphadenectomy was performed at Berg axillary levels I and II in all the patients. The mean number of lymph nodes
removed, including the SLN, was 13.2 (typical deviation: 4.4) with
a range of 3–24 lymph nodes. The mean time from the date of the
last NCT cycle to surgery was of 38.4 natural days (typical deviation:
11.3) with a minimum of 21 and a maximum of 80 days.
Table 2 shows the results of the SLN and the pathological axillary
status in both the total population and the groups of patients with
clinically/ultrasonographically axilla-negative (cN0) or -positive
(cN1) patients at diagnosis. Table 3 depicts the main parameters
of diagnostic validity of SLNB in the patients treated with NCT with
the estimation of the CI 95%, percentage of identification of the
SLN, rate of FN, NPV and diagnostic precision. In our experience, on
Documento descargado de http://www.elsevier.es el 01/10/2016. Copia para uso personal, se prohíbe la transmisión de este documento por cualquier medio o formato.
120
Á.C. Rebollo-Aguirre et al. / Rev Esp Med Nucl Imagen Mol. 2012;31(3):117–123
Of the three patients with FN results of the SLN, one corresponded to the cN0 group and two to the cN1 without
anatomopathologic confirmation of axillary status. In the 24 cN1
patients with confirmation of axillary status the SLN was identified
in 79.2% of the cases with no FN results.
The definitive anatomopathologic study after surgery showed
pCR to NCT in the primary tumor in 25 patients (28.4%). In 54.5%
of the cases the axilla was negative in the final pathologic examination. Only 14.8% of the patients showed pCR in both the primary
tumor and the axilla.
On univariate analysis the percentage of identification of the
SLN was significantly lower in cN1 compared with cN0 patients
(83.8 and 98%, respectively, P = 0.015). In the remaining variables
studied no statistically significant differences were found in either
SLN identification or the rate of FN (Table 4).
On comparing the number of axillary lymph nodes removed in
the patients who had received NCT and in women in the SLNB
validation phase with early stage breast cancer without primary
systemic treatment, no statistically significant differences were
found in the mean number of SLN identified, the mean number of
axillary lymph nodes resected or the number of patients with less
than 10 lymph nodes removed in the lymphadenectomy (Table 5).
Table 1
Clinical and pathological characteristics of the patients.
Characteristics
NCT
n (%)
Without treatment16
n (%)
Patients
88
64
Age (years)
Mean (range)
49.4 (31–68)
56.5 (26–79)
Menopausal status
Premenopausal
50 (56.8)
29 (45.3)
Tumor localization
Right breast
UEQ
47 (53.4)
53 (60.2)
33 (51.4)
40 (62.5)
Tumor size (mm)
Mean (range)
35.5 (18–60)
17.9 (6–30)
Histological type
Infiltrating ductal
69 (78.4)
55 (85.9)
Hormone receptors
Estrogen
Progesterone
65 (73.9)
56 (63.6)
52 (59.1)
54 (61.4)
HER-2/neu expression
Ki-67 ≥ 20% expression
22 (25.0)
32 (36.3)
9 (14.1)
19 (29.7)
2 (2.3)
78 (88.6)
8 (9.1)
39 (61.9)
25 (38.1)
–
51 (58.0)
37 (42.0)
64 (100)
–
–
48 (54.6)
37 (42.0)
3 (3.4)
39 (61.9)
25 (38.1)
–
–
TNM classification
Primary tumor
T1
T2
T3
Lymph nodes
N0
N1
Stages
I
II-A
II-B
III-A
Discussion
On analyzing the results obtained in our group of patients we
observed that both the percentage of SLN identification and the
rate of FN did not achieve the classically recommended values in
the process of SLNB validation (rate of at least 95% identification of
SLN with a rate of FN of 5% or less).4 In our population, the SLN was
identified in 92% of the cases with a rate of FN of 8.3%. Nonetheless,
these values are similar to those reported in the literature in the
usual clinical conditions in both women with early stage breast
cancer and in those treated with primary systemic chemotherapy.
Classe et al.17 obtained a percentage of SLN identification of
90.2% and a rate of FN of 11.5%. In our country, Duch et al.18 prospectively evaluated post-NCT SLNB in 30 patients with breast cancer
in stages T2–3, N0–1, M0 before the initiation of treatment, with a
rate of SLN detection of 90% and a rate of FN of 9%. In the systematic reviews of Xing et al.,9 Kelly et al.10 and van Deurzen et al.11
the percentages of SLN identification were estimated to be 90%
(CI 95%: 88–91%), 89.6% (CI 95%: 86.0–92.3%) and 90.9% (CI 95%:
88.0–93.1%) with a rate of FN of 12% (CI 95%: 9–16%), 8.4% (CI
95%: 6.4–10.9%) and 10.5% (CI 95%: 8.1–13.6%), respectively. In the
69 articles including 8059 patients with early stage breast cancer
included in the meta-analysis by Kim et al.19 the mean percentage
UEQ: upper external quadrant; NCT: neoadjuvant chemotherapy.
validating the technique in patients with early stage breast cancer
without primary systemic treatment, the SLN has been identified
in 96.9% of the cases with a rate of FN of 4.3%.16
The results of the anatomopathologic study of the SLN were
the following: 46 negative, 2 ITC, 20 micrometastasis and 13
macrometastasis. In 51.5% of the women with positive results all
the SLN identified were affected. Axillary involvement of the SLN
identified was only present in 69.4% of the patients (17 micrometastasis and 8 macrometastasis). Other axillary adenopathies showed
metastasis in 15% of the women with micrometastasis and 38.5%
of those with macrometastasis in the SLN.
Table 2
Contingency tables: results of sentinel lymph node and pathological axillary status.
Total population
SLN (+)
SLN (−)
N0
pN (+)
pN (−)
33
3
0
45
SLN (+)
SLN (−)
N1
pN (+)
pN (−)
19
2
0
29
SLN (+)
SLN (−)
pN (+)
pN (−)
14
1
0
16
cN: clinical/ultrasonographic axilla at diagnosis; SLN: sentinel lymph node; pN: pathological axillary status.
Table 3
Parameters of diagnostic validity of scintigraphic detection of the sentinel lymph node.
Identification
Total
cN0
cN1
FN
NPV
Precision
n
%
n
%
n
%
CI 95%
n
%
CI 95%
81/88
50/51
31/37
92.0
98.0
83.8
3/36
2/21
1/15
8.3
9.5
6.7
45/48
29/31
16/17
93.7
93.6
94.1
86–100
83–100
80–100
78/81
48/50
30/31
96.3
96.0
96.8
91–100
90–100
89–100
cN: clinical/ultrasonographic axilla at diagnosis; FN: false negative; CI 95% confidence interval of 95%; NPV: negative predictive value.
Documento descargado de http://www.elsevier.es el 01/10/2016. Copia para uso personal, se prohíbe la transmisión de este documento por cualquier medio o formato.
Á.C. Rebollo-Aguirre et al. / Rev Esp Med Nucl Imagen Mol. 2012;31(3):117–123
Table 4
Correlation between clinicopathological factors and parameters of diagnostic
validity.
Identification of
SLN (81/88)
False negative
(3/36)
n
Pa
n
Pb
Age
≤60 years
>60 years
72/78
9/10
0.800
3/32
0/4
0.477
Menopausal status
Premenopausal
Postmenopausal
46/50
35/38
0.986
1/20
2/16
0.217
73/78
8/8
0.383
3/31
0/5
0.468
62/68
19/20
0.579
1/21
2/15
0.359
49/53
32/35
0.862
1/32
2/14
0.303
Clinical axillary status at diagnosis
Negative
50/51
Positive
31/37
0.015
2/22
1/14
0.503
Histological type
Infiltrating ductal
Other histological type
63/69
18/19
0.624
3/27
0/9
0.230
Clinical response
Complete
Not complete
35/38
46/50
0.986
0/11
3/25
0.229
Pathological response
Complete
Not complete
23/25
58/63
0.992
1/10
2/26
0.822
Tumor size
Initial
T1–T2
T3
After treatment
T0–T1
T2–T3
Tumor localization
Upper external quadrant
Other quadrants
SLN: sentinel lymph node.
a
Pearson Chi-square test.
b
Fisher exact text. Statistical significance with P < 0.05.
of SLN identification was 89% (CI 95%: 86.0–92.0%) and the rate of
FN was 8.4% (CI 95%: 6.8–10.0%).
Axillary status at diagnosis is the most controversial clinical
variable in the literature in relation to the diagnostic precision of
SLNB in patients treated with NCT, and most of the studies include
patients with clinically negative axilla at diagnosis.13 Hunt et al.20
analyzed the results of SLNB in 3746 patients with T1–T3 and clinically negative axilla breast cancer 575 of whom had received NCT.
On adjusting the results for clinical stage no differences were found
in locoregional recurrence, disease-free survival or overall survival
between the two groups of patients.
In our experience the percentage of SLN identification in the
cN0 patients was 98% and 83.8% in the cN1 patients (P = 0.015),
Table 5
Comparison of the number of axillary lymph nodes resected.
Sentinel lymph node
Mean ± SD
Range
NCT
Without treatment (n = 64)
P
1.7 ± 0.8
(1–5)
1.6 ± 0.9
(1–6)
0.332a
14.6 ± 5.8
(3–33)
23.4%
20.3%
0.103a
Axillary lymphadenectomy
Mean ± SD
13.2 ± 4.4
Range
(3–24)
< 10 lymph nodes
20.4%
≥20 lymph nodes
5.7%
0.533b
0.008b
NCT: neoadjuvant chemotherapy.
a
Student’s t-test for independent samples.
b
Mann–Whitney U-test; SD: standard deviation. Statistical significance with
P < 0.05.
121
with no significant differences in the rate of FN (9.5 and 6.7%,
respectively; P = 0.503). In 6 of the 7 patients in whom the SLN
was not identified, the axilla was cN1 prior to initiating treatment. The diagnostic precision of the SLNB was similar in both
groups: cN0: 96% (CI 95%: 89.6–100%); and cN1: 96.8% (CI 95%:
88.9–100%). These results coincide with those of other authors who
reported that a positive axillary status at diagnosis diminished the
capacity of SLN identification but did not modify its diagnostic precision. Lee et al.21 studied 875 patients 219 of whom had received
NCT for presenting positive axillary lymph nodes at diagnosis in
either the clinical examination or by imaging techniques. The percentage of SLN identification was significantly lower in the group
receiving chemotherapy compared with the group which did not
undergo previous treatment 77.6% and 97%, respectively (P < 0.001).
To the contrary, the rate of FN did not show significant differences
between the patients with NCT (5.6%) and those without (7.4%)
(P = 0.681).
In our case, on analyzing the criteria of evaluation and the
standards of quality of the self-examination guidelines of the
Spanish Society of Senology and Breast Disease (SESPM),22 of
the three operative criteria considered essential for implementing SLNB post-NCT in the health care process for breast cancer,
the sensitivity accredited corresponds to level I of the standard
(90–95%, fulfill the minimum requisites, but the results can be
markedly improved); the technical efficacy of SLN detection is
associated with level II in the total population (85-95%, sufficiently fulfill the minimum requisites required), level III in N0
patients (>95%, completely fulfill the requisites) and level 0 in
the N1 patients (<85%, do not fulfill the minimum requisites
required); and the average axillary SLN per patient is related to
level II (<2.2).
Based on these results and review of the literature, although
it is true that the lower percentage of SLN identification in the
axilla-positive patients would not avoid lymphadenectomy in
these cases, intraoperative analysis of the SLN preserves its diagnostic validity for decision making in relation to the surgical
treatment and the staging of the axilla. In our series axillary lymphadenectomy could have been avoided in 45 patients with a truly
negative SLN result (51.1% of the cases). In addition, 69.4% of the
axilla-positive patients in the definitive anatomopathologic study,
the SLN was the only lymph node affected. The performance of
SLNB after NCT has the advantages that it allows identification
of patients with axillary pCR (20–40% of the cases), it provides
prognostic information for the selection of adjuvant therapies
and reduces the number of unnecessary axillary lymphadenectomies, maintaining the conventional surgical sequence after
chemotherapy.12,13
For years it has been considered that NCT produces a
reduction in the number of lymph nodes resected in axillary
lymphadenectomy.23 In our patients the NCT did not produce a
reduction in the mean number of lymph nodes removed in the
lymphadenectomy, in the proportion of emptying with 10 or more
axillary lymph nodes or in the number of SLN identified during
surgery. Straver et al.24 analyzed the results of lymphadenectomy
in 191 women after NCT and 192 women undergoing primary
surgery and axillary lymphadenectomy after one positive SLN.
There were no differences between the two groups in the mean
number of lymph nodes resected, 16.3 and 15.8 (P = 0.4) or in the
proportion of patients with a retrieval of less than 10 lymph nodes
(7 and 6%, P = 0.7).
In our case we used the perioareolar injection for the administration of the radiotracer. One of the advantages of this technique
is that the areola is rich in lymph vessels thereby facilitating the
uptake of the radiotracer. Another possible advantage is that the
tissue surrounding the areola is less likely to be affected by the secondary changes to NCT, provided that this is not the localization of
Documento descargado de http://www.elsevier.es el 01/10/2016. Copia para uso personal, se prohíbe la transmisión de este documento por cualquier medio o formato.
122
Á.C. Rebollo-Aguirre et al. / Rev Esp Med Nucl Imagen Mol. 2012;31(3):117–123
the primary tumor.25 In patients treated with NCT the superficial
injection techniques may be greater those of deep injection since
the response of the primary tumor to chemotherapy may alter or
interrupt normal lymphatic drainage thereby hindering the uptake
and migration of the radiotracer when deep routes of administration are used.26,27
This study has several methodological limitations. The first is
related to axillary staging at diagnosis: although physical examination and axillary ultrasonography were performed in all the
patients, pathological confirmation of axillary status was only
obtained in 64.9% of the cN1 cases. There is currently consensus
on the need to precisely know the lymph node status in women
with breast cancer prior to performing SLNB, and axillary evaluation should include palpation and ultrasound-guided puncture
of the suspicious lymph nodes.28 Thus, most protocols predetermine the interval between NCT and surgery as a maximum of
4–6 weeks, coinciding with the time of chemotherapy recuperation and maximum tumoral reduction, and recommend a level of
compliance with this indicator of 90%.23 In our series only 79.5%
of the patients underwent surgery within 42 days after the finalization of chemotherapy. Moreover, two procedures were used
for the anatomopathological study of the SLN. The great advantage of the implementation of the OSNA procedure is probably
that of achieving standardization of highly sensitive and specific
results in order to compare really similar prognostic groups that do
not depend on either the procedure of SLN study or the pathologist doing it.29 Lastly, the process of validation has limitations
since axillary lymphadenectomy must be completed for its determination, thereby invalidating the practical use of SLNB, and its
permanent nature does not allow periodical evaluation. New indicators of quality should be elaborated and applied to evaluate
the aspects related to SLNB without the need to perform axillary
lymphadenectomy.30
Conclusions
In patients with breast cancer treated with NCT the use
of SLNB with periareolar injection of albumin nanocolloid is a
technically feasible procedure which fulfills the operative criteria essential for the implementation of SLNB in the health
care process for breast cancer according to the self-examination
guidelines of the SESPM, despite not achieving the classically
recommended parameters in the process of SLNB validation.
In our patients NCT did not reduce either the number of SLN
identified or the axillary lymph nodes removed in the axillary
lymphadenectomy. We consider that the inclusion of SLNB postNCT in the systematic algorithms of the management of patients
with breast cancer who are candidates for primary systemic therapy conduces to an important reduction in unnecessary axillary
lymphadenectomies
Financial support
This study was financially supported by the Consejería de Salud
de la Junta de Andalucía, number PI-0080/2007, subventions for
financing Biomedical Investigation and Health Sciences in Andalusia.
Conflict of interest
The authors declare no conflict of interest.
References
1. Instituto de Salud Carlos III. La situación del cáncer en España. Madrid: Ministerio
de Sanidad y Consumo; 2005.
2. Sabadell Mercadal MD, Piñero Madrona A, Giménez Climent MJ. Tratamiento
quirúrgico. Cirugía primaria. Cirugía en neoadyuvancia. Ganglio centinela. In:
Modolell Roig A, Sabadell Mercadal MD, Izquierdo Sanz M, Prats de Puig M,
editors. Manual de Práctica Clínica en Senología. Valencia: Sociedad Española
de Senología y Patología Mamaria; 2010. p. 48–51.
3. Lyman GH, Giuliano AE, Somerfield MR, Benson AB, Bodurka DC, Burstein
HJ, et al. American Society of Clinical Oncology guideline recommendations
for sentinel lymph node biopsy in early-stage breast cancer. J Clin Oncol.
2005;23:7703–20.
4. Piñero A, Giménez J, Merck B, Vázquez C, Grupo de Expertos. Reunión de consenso sobre la biopsia selectiva del ganglio centinela en el cáncer de mama.
Sociedad Española de Senología y Patología Mamaria. Rev Esp Med Nucl.
2007;26:176–80.
5. Buscombe J, Paganelli G, Burak ZE, Waddington W, Maublant J, Prats E, et al. Sentinel node in breast cancer procedural guidelines. Eur J Nucl Med Mol Imaging.
2007;34:2154–9.
6. Vidal-Sicart S, Rioja Martín ME. Detección gammagráfica e intraoperatoria
del ganglio centinela en el cáncer de mama. Rev Esp Med Nucl. 2009;28:
41–3.
7. Mieog JSD, van der Hage JA, van de Velde CJH. Quimioterapia preoperatoria para
mujeres con cáncer de mama operable (Revisión Cochrane traducida). In: La
Biblioteca Cochrane Plus. Oxford: Update Software Ltd.; 2008. Available from:
http://www.update-software.com. (Traducida de The Cochrane Library, 2008
Issue 2. Chichester, UK: John Wiley & Sons, Ltd.).
8. Insa A, Chirivella I, Lluch A. Tratamiento neoadyuvante del cáncer de mama
operable. Med Clin (Barc). 2006;126:295–303.
9. Xing Y, Foy M, Cox DD, Kuerer HM, Hunt KK, Cormier JN. Meta-analysis of sentinel
lymph node biopsy after preoperative chemotherapy in patients with breast
cancer. Br J Surg. 2006;93:539–46.
10. Kelly AM, Dwamena B, Cronin P, Carlos RC. Breast cancer: sentinel node identification and classification after neoadjuvant chemotherapy—systematic review
and meta analysis. Acad Radiol. 2009;16:551–63.
11. van Deurzen CHM, Vriens BEPJ, Tjan-Heijen VCG, van der Wall E, Albregts M,
van Hilligersberg R, et al. Accuracy of sentinel node biopsy after neoadjuvant
chemotherapy in breast cancer patients: a systematic review. Eur J Cancer.
2009;45:3124–30.
12. Piñero A, Giménez J, Vidal-Sicart S, Intra M. Selective sentinel lymph node biopsy
and primary systemic therapy in breast cancer. Tumori. 2010;96:17–23.
13. Sabel MS. Sentinel lymph node biopsy before or after neoadjuvant chemotherapy: pros and cons. Surg Oncol Clin N Am. 2010;19:519–38.
14. Solá M, Fraile M. Ganglio centinela y neoadyuvancia en cáncer de mama: en
busca del mejor escenario. Rev Esp Med Nucl. 2010;29:316–8.
15. Muñoz M, Pahisa J, Caparrós X, Vidal-Sicart S. Ganglio centinela y neoadyuvancia
en cáncer de mama. Rev Esp Med Nucl. 2010;29:319–20.
16. González Perán E, Ramos Font C, González Salmerón MD, Rebollo Aguirre AC,
Menjón Beltrán S, Llamas Elvira JM. Validación de la técnica de biopsia selectiva
del ganglio centinela (BSGC) en pacientes con cáncer de mama. Cienc Ginecol.
2006;10 Suppl. 1:81.
17. Classe J-M, Bordes V, Campion L, Mignotte H, Dravet F, Leveque J, et al. Sentinel lymph node biopsy after neoadjuvant chemotherapy for advanced breast
cancer: results of Ganglion Sentinelle et Chimiothérapie Neoadjuvante, a french
prospective multicentric study. J Clin Oncol. 2009;27:726–32.
18. Duch Renom J, Estorch Cabrera M, Rodríguez Revuelto AA, Rivera Codías E,
Artigas Guix C, Camacho Martí V, et al. Biopsia selectiva del ganglio centinela
postquimioterapia neoadyuvante en pacientes con cáncer de mama. Rev Esp
Med Nucl. 2010;29 Suppl. 1:7.
19. Kim T, Giuliano AE, Lyman GH. Lymphatic mapping and sentinel lymph node
biopsy in early-stage breast carcinoma. A metaanalysis. Cancer. 2006;106:4–16.
20. Hunt KK, Yi M, Mittendorf E, Guerrero C, Babiera GV, Bedrosian I, et al. Sentinel lymph node surgery after neoadjuvant chemotherapy is accurate and
reduces the need for axillary dissection in breast cancer patients. Ann Surg.
2009;250:558–66.
21. Lee S, Kim EY, Kang SH, Kim SW, Kim S-K, Kang KW, et al. Sentinel node identification rate, but not accuracy, is significantly decreased after pre-operative
chemotherapy in axillary node-positive breast cancer patients. Breast Cancer
Res Treat. 2007;102:283–8.
22. Fraile M, Giménez J. Criterios de evaluación y estándares de calidad. In: Reunión
de consenso sobre la biopsia selectiva del ganglio centinela en el cáncer de
mama. Madrid: Sociedad Española de Senología y Patología Mamaria; Marzo
2007. Available from: http://www.sespm.es/cursos/consenso bgc cm.php.
23. Saura RM, Gimeno V, Blanco MC, Colomer R, Serrano P, Acea B, et al. Desarrollo
de indicadores de proceso y resultado y evaluación de la práctica asistencial
oncológica. Madrid: Plan de Calidad para el Sistema Nacional de Salud. Ministerio de Sanidad y Consumo. Agència d’Avaluació de Tecnologia i Recerca
Mèdiques de Cataluña; 2007. Informes de Evaluación de Tecnologías Sanitarias,
AATRM núm. 2006/02.
24. Straver ME, Rutgers EJT, Oldenburg HSA, Wesseling J, Linn SC, Russell NS,
et al. Accurate axillary lymph node dissection is feasible after neoadjuvant
chemotherapy. Am J Surg. 2009;198:46–50.
25. Shimazu K, Tamaki Y, Taguchi T, Akazawa K, Inoue T, Noguchi S. Sentinel node
biopsy using periareolar injection of radiocolloid for patients with neoadjuvant
chemotherapy-treated breast carcinoma. Cancer. 2004;100:2555–61.
26. Kinoshita T. Sentinel lymph node biopsy is feasible for breast cancer patients
after neoadjuvant chemotherapy. Breast Cancer. 2007;14:10–5.
27. Abollahi A, Jangjoo A, Dabbagh Kakhki VR, Zakavi SR, Memar B, Naser Forghani
M, et al. Factors affecting sentinel lymph node detection failure in breast
Documento descargado de http://www.elsevier.es el 01/10/2016. Copia para uso personal, se prohíbe la transmisión de este documento por cualquier medio o formato.
Á.C. Rebollo-Aguirre et al. / Rev Esp Med Nucl Imagen Mol. 2012;31(3):117–123
cancer patients using intradermal injection of the tracer. Rev Esp Med Nucl.
2010;29:73–7.
28. Chung A, Giuliano A. Axillary staging in the neoadjuvant setting. Ann Surg Oncol.
2010;17:2401–10.
29. Bernet L, Cano R, Martínez-Benaclocha M, Dueñas B, Matias-Guiu X, Morell Ll,
et al. Ganglio centinela en cáncer de mama: ¿histológico o molecular? Estudio
123
diagnóstico comparativo multicéntrico español. Grupo de Estudios Senológicos.
Rev Senología Patol Mam. 2010;23:3–7.
30. Wells BJ, Najjar H, Wright FC, Holloway CMB, Fraser N, McCready D, et al.
Measuring the quality of sentinel lymph node biopsy in breast cancer using
newly developed quality indicators: a feasibility study. Ann Surg Oncol.
2011;18:78–85.