129 Pre-Physical Possible - Physics Education Research
advertisement
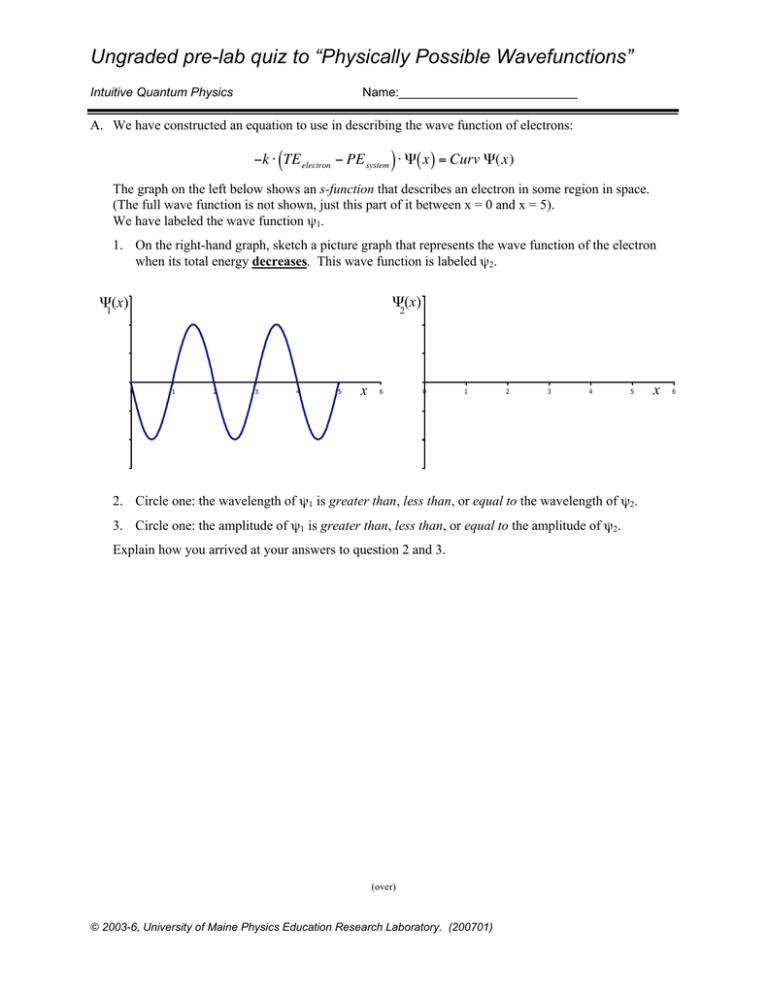
Ungraded pre-lab quiz to “Physically Possible Wavefunctions” Intuitive Quantum Physics Name:__________________________ A. We have constructed an equation to use in describing the wave function of electrons: −k ⋅ (TE electron − PE system ) ⋅ Ψ( x ) = Curv Ψ(x) The graph on the left below shows an s-function that describes an electron in some region in space. (The full wave function is not shown, just this part of it between x = 0 and x = 5). We have labeled the wave function ψ1. € 1. On the right-hand graph, sketch a picture graph that represents the wave function of the electron when its total energy decreases. This wave function is labeled ψ2. Ψ(x) 2 Ψ(x) 1 0 1 2 3 4 5 x 6 0 1 2 3 4 5 2. Circle one: the wavelength of ψ1 is greater than, less than, or equal to the wavelength of ψ2. 3. Circle one: the amplitude of ψ1 is greater than, less than, or equal to the amplitude of ψ2. Explain how you arrived at your answers to question 2 and 3. (over) © 2003-6, University of Maine Physics Education Research Laboratory. (200701) x 6 p.2 B. Describe the relationship between electrons and atoms in as much detail as possible. Write as much as you can. Answer questions including • “What makes an atom?” • “Where is the electron in the atom?” • “How is an electron in an atom different from an electron moving far away from an atom?” plus any others that you wish to address.







