Chapter - 9 CORE-SHELL NANOPARTICLES
advertisement

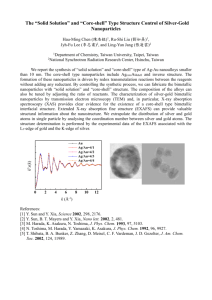
Chapter - 9 CORE-SHELL NANOPARTICLES Fig. 9.1: Transmission electron micrographs of silica coated gold nanoparticles. The shell thicknesses are (a) 10 nm, (b) 23 nm, (c) 58 nm, and (d) 83 nm. Reprinted with permission from Liz-Marzan, et al. (L. M. Liz-Marzan, M. Giersig, P. Mulvaney, Langmuir, 1996, 12, 4329.). Copyright (1996) American Chemical Society. Fig. 9.2: TEM image of ZrO2 coated Ag nanoparticles. Reprinted with permission from Tom, et al. (R. T. Tom, A. S. Nair, N. Singh, M. Aslam, C. L. Nagendra, R. Philip, K. Vijayamohanan, T. Pradeep, Langmuir, 2003, 19, 3439). Copyright (2003) American Chemical Society. Fig. 9.3: TEM image of Pt-maghemite core-shell nanoparticles having different shell thickness made with different shell-forming precursors. The shell thicknesses are 3.5 nm and 5.4 nm, respectively (left to right). Reprinted with permission from Teng, et al. ( X. Teng, D. Black, N. J. Watkins, Y. Gao, H. Yang, Nano Lett., 2003, 3, 261). Copyright (2003) American Chemical Society. Fig. 9.4: HRTEM images of Ag@ZrO2 core-shell nanoparticles functionalized with a stearate monolayer. From Nair et. al.( A. S. Nair, T. Pradeep, I. MacLaren, J. Mater. Chem., 2004, 14, 857.). Reproduced with permission from the Royal Society of Chemistry. Fig. 9.5: TEM images of Gold-silica inverse opals. The core dimension is ~15 nm and the silica shells are around 8, 18 and 28 nm, respectively. Scale bars are 50 nm in all cases. Reprinted from ( D. Wang, V. Salgueirino-Maceira, L. M. Liz-Marzan and F. Caruso, Adv. Mater., 2002, 14, 908.). Copyright (2002) Wiley-VCH. Fig. 9.6: TEM images of Au nanoparticles (left) coated with Pt (right) in the ratio 1:2. Reprinted with permission from Henglein. ( A. Henglein, J. Phys. Chem. B, 2000, 104, 2201.). Copyright (2000) American Chemical Society. Fig. 9.7: TEM of polypyrrole coated SiO2 core-shell nanoparticles. Reproduced from ( C. L. Huang, E. Matijevic, J. Mater. Res., 1995, 10, 1327.). Copyright 1995 Materials Research Society, also published in F. Caruso, Adv. Mater., 2001, 13, 11. Copyright (2001) Wiley-VCH. Fig. 9.8: TEM of polypyrrole-capped Au nanoparticles (left) and with further increase in shell thickness by polymerization with poly (N-methylpyrrole). Reprinted with permission from Marinakos, et al. ( S. M. Marinakos, J. P. Novak, L. C. Brousseau, A. B. House, E. M. Edeki, J. C. Feldhaus, D. L. Feldheim, J. Am. Chem. Soc., 1999, 121, 8518.). Copyright (1999) American Chemical Society. Fig. 9.9: TEM of LbL assembled polystyrene-capped Au nanoparticles. Reproduced from F. Caruso, Adv. Mater., 2001, 13, 11. Copyright (2001) Wiley-VCH. Fig. 9.10: Powder XRD pattern of Pt@Fe2O3 core-shell nanoparticles. Reprinted with permission from Teng, et al. ( X. Teng, D. Black, N. J. Watkins, Y. Gao, H. Yang, Nano Lett., 2003, 3, 261.). Copyright (2003) American Chemical Society. Fig. 9.11: Cyclic voltammetry responses of core-shell nanosystems. Reprinted from ( D. S. Koktysh, X. Liang, B.-G. Yun, I. Pastoriza-Santos, R. L. Matts, M. Giersig, C. Serra-Rodriguez, L. M. Liz-Marzan, N. A. Kotov, Adv. Funct. Mater., 2002, 12, 255.) and ( R. T. Tom, A. S. Nair, N. Singh, M. Aslam, C. L. Nagendra, R. Philip, K. Vijayamohanan, T. Pradeep, Langmuir, 2003, 19, 3439.), respectively. Copyrights (2002) Wiley-VCH and (2003) American Chemical Society, respectively. Fig. 9.12: Surface plasmon resonance in metal nanoparticles in an electromagnetic field. The displacement of the conduction band electrons relative to the nuclei can be seen. Reprinted with permission from Kelly, et al. ( K. L. Kelly, E. Coronado, L. L. Zhao, G. C. Schatz, J. Phys. Chem. B, 2003, 107, 668.). Copyright (2003) American Chemical Society. Fig. 9.13: Dispersion diagram showing the conditions of surface plasmon resonance absorption as a function of the wavelength of the incident light. Reproduced from ( P. Mulvaney, L. M. LizMarzan, M. Giersig, T. Ung, J. Mater. Chem., 2000, 10, 1259 ). Reproduced with permission from the Royal Society of Chemistry. Fig. 9.14: The absorption spectra of Au@SiO2 colloids as a function of solvent refractive index (top figures) and Ag@TiO2 (bottom A) and Ag@ZrO2 (bottom B) as a function of core dimension (A) and shell thickness (B). Reprinted with permission from Liz-Marzan, et al. ( L. M. Liz-Marzan, M.Giersig, P. Mulvaney, Langmuir, 1996, 12, 4329.). (top) and Tom, et al.6 (bottom). Copyright (1996 and 2003, respectively) American Chemical Society. Fig. 9.15: Transmitted and reflected colors from Au@SiO2 multilayer thin films with varying silica shell thickness. Reprinted with permission from ( T. Ung, L. M. Liz-Marzan, P. Mulvaney, J. Phys. Chem. B, 2001, 105, 3441). Copyright (2001) American Chemical Society. Fig. 9.17: TEM images of Ag@TiO2 core-shell nanoparticles (a) and the TiO2 nanoshells formed from the same after leaching the core with NH3. The inset of figure b shows an expanded view of a TiO2 shell. Reprinted from ( D. S. Koktysh, X. Liang, B.-G. Yun, I. Pastoriza-Santos, R. L. Matts, M. Giersig, C. Serra-Rodriguez, L. M. Liz-Marzan, N. A. Kotov, Adv. Funct. Mater., 2002, 12, 255). Copyright (2002) Wiley-VCH. Fig. 9.18: Schematic showing the stability of core-shell nanoparticles with intense laser fluences. Fig. 9.19: Optical limiting responses of Au@TiO2 and Au@ZrO2 core-shell nanoparticles using the Z-scan technique. Inset shows the Z-scan curve of Ag@ZrO2 system. Reprinted with permission from ( R. T. Tom, A. S. Nair, N. Singh, M. Aslam, C. L. Nagendra, R. Philip, K. Vijayamohanan, T. Pradeep, Langmuir, 2003, 19, 3439). Copyright (2003) American Chemical Society.