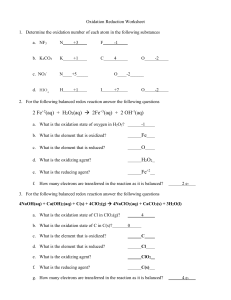
Balancing Redox Reactions Dr. Landrum The half
advertisement

Balancing Redox Reactions Dr. Landrum The half-reaction method for balancing redox reactions is an approach which breaks the process into steps that are each simple and easily checked. To achieve success with this method it is absolutely essential to memorize the steps and apply them IN ORDER. This process works every time when applied as written. It will fail, every time, if you don’t do each step IN ORDER. Check your work! Most reactions occur in aqueous solution. These reactions will be carried out under acidic or basic conditions, you will be told which, do not guess! 1) The first job in balancing a redox reaction is to determine the oxidation number of every species present in the reaction. Rules for determining oxidation numbers are attached. 2) Eliminate spectator ions. 3) The oxidation numbers of one element will increase (oxidation) and those of another element will decrease (reduction). Write the oxidation step and reduction step as two separate reactions. Balance the element undergoing the reduction or oxidation and include the appropriate number of electrons (be sure you put them on the correct side!) 4) Balance the charge of each half-reaction by adding H+ for acidic solution, OH- for basic solution. 5) Balance oxygen by adding H2O in the correct numbers to each half-reaction. 6) Check that the number of hydrogen atoms is now balanced. If not, you must return to step one! (just like Monopoly!) 7) Multiply each half-reaction by the number of electrons present in the other halfreaction and add the two half-reactions. 8) The resulting reaction will have some species that are present on both sides of the reaction. Cancel the appropriate number leaving each species on only one side of the reaction. 9) Add back spectator ions so that each side of the reaction has no charge. 10) Check your work! Example: Balance Acidic Media FeSO4 + KMnO4 1) determine oxidation numbers Fe S O K Mn O 2+ 6+ 2- à 1+ 7+ 2- Fe2(SO4)3 + MnSO4 Fe S O 3+ 6+ 2- Mn S O 2+ 6+ 2- Fe and Mn change oxidation state; S, O, and K do not. 2) Eliminate K+, SO423) Write an unbalanced, net ionic equation, by deleting the spectator ions. Fe2+ + MnO4- à Fe3+ + Mn2+ 4) Write the oxidation and reduction half-reactions. Be sure to balance the redox active element and include the correct number of electrons. Fe2+ à Fe3+ + 5e- + MnO4- à 6- e- (Charge balanced) Mn2+ 2+ (Charge imbalanced) 5) Balance the charges (with H+ because this is in acidic solution) 5e- + 8H+ + MnO4- à Mn2+ (Charge balanced) 6) Balance O with H2O 5e- + 8H+ + MnO4- à Mn2+ + 4H2O Hydrogen is Balanced!!! 7) Add half reactions after cross multiplying by the number of electrons in each 5x (Fe2+ à Fe3+ + e-) + Mn2+ + 4H2O 5e + 8H + MnO4 à 5e- + 8H+ + MnO4- + 5Fe2+ à Mn2+ + 5Fe3+ + 5e- + 4H2O 8) Cancel those species that remain unchanged (in this case only e-, but often we will have H2O, H+, or OH- on both sides) 8H+ + MnO4- + 5Fe2+ à Mn2+ + 5Fe3+ + 4H2O this is a suitably balanced net ionic equation! 9) Add back the K+ and SO42- 4H2SO4 + KMnO4 + 5FeSO4 à MnSO4 +5/2 Fe2(SO4)3 + K+ + 1/2SO42-+ 4H2O Multiply by 2 to eliminate the fractional coefficients 8H2SO4 + 2KMnO4 + 10FeSO4 à 2MnSO4 + 5Fe2(SO4)3 + K2SO4+ 4H2O Balanced – Determining oxidation numbers: All alkali metal ions in salts are 1+ All alkaline earth ions in salts are 2+ Acidic protons are 1+ Halides are always 1- (except when bonded to O or F) Oxygen 2- (except when bonded to another oxygen ) The assign the oxidation numbers that are known from the above list. Assign a charge to any remaining atom so that the net charge on every molecule or salt is zero. Example: K2Cr2O7 à K = 1+; O = 27 x 2- + 2 x 1+ = 122 x Cr + 12- = 0 therefore Cr = 6+ N2O4 à O = 24 x 2- = 88- + 2 x N = 0 therefore N = 4+ H2O2 à H = 1+ 2 x 1+ = 2+ 2+ + 2 x O = 0 therefore O = 1- (Oxygen is bonded to oxygen! Peroxide)