Dung and nest surveys: estimating decay rates - CREEM
advertisement

Journal of Applied
Ecology 2003
40, 1102 –1111
METHODOLOGICAL INSIGHTS
S.
M
Estimating
ethodological
E.
Laing
decay
et al.Insights
rates
Blackwell
Oxford,
Journal
JAPPL
British
0021-8901
612
40
2003
Ecological
of
UK
Publishing
Applied
Society,
Ecology
Ltd. 2003
Dung and nest surveys: estimating decay rates
S. E. LAING*†, S. T. BUCKLAND‡, R. W. BURN§, D. LAMBIE¶
and A. AMPHLETT**
†School of Applied Statistics, University of Reading, Reading, UK; ‡Centre for Research into Ecological and
Environmental Modelling, University of St Andrews, St Andrews, UK; §Statistical Services Centre, University of
Reading, Reading, UK; ¶Tomdhu Cottage, Boat of Garten, Inverness-shire, UK; and **Royal Society for the
Protection of Birds, Forest Lodge, Nethy Bridge, Inverness-shire, UK
Summary
1. Wildlife managers often require estimates of abundance. Direct methods of estimation are often impractical, especially in closed-forest environments, so indirect methods
such as dung or nest surveys are increasingly popular.
2. Dung and nest surveys typically have three elements: surveys to estimate abundance
of the dung or nests; experiments to estimate the production (defecation or nest construction) rate; and experiments to estimate the decay or disappearance rate. The last of
these is usually the most problematic, and was the subject of this study.
3. The design of experiments to allow robust estimation of mean time to decay was
addressed. In most studies to date, dung or nests have been monitored until they disappear. Instead, we advocate that fresh dung or nests are located, with a single followup visit to establish whether the dung or nest is still present or has decayed.
4. Logistic regression was used to estimate probability of decay as a function of time,
and possibly of other covariates. Mean time to decay was estimated from this function.
5. Synthesis and applications. Effective management of mammal populations usually
requires reliable abundance estimates. The difficulty in estimating abundance of mammals in forest environments has increasingly led to the use of indirect survey methods,
in which abundance of sign, usually dung (e.g. deer, antelope and elephants) or nests (e.g.
apes), is estimated. Given estimated rates of sign production and decay, sign abundance
estimates can be converted to estimates of animal abundance. Decay rates typically vary
according to season, weather, habitat, diet and many other factors, making reliable
estimation of mean time to decay of signs present at the time of the survey problematic.
We emphasize the need for retrospective rather than prospective rates, propose a strategy
for survey design, and provide analysis methods for estimating retrospective rates.
Key-words: decay rate experiments, indirect surveys, sign surveys.
Journal of Applied Ecology (2003) 40, 1102–1111
Introduction
To manage wild mammal populations effectively, information is needed on abundance and on factors that affect
abundance over time. A wide range of methods exists for
direct surveys of animals (Seber 1982; Borchers, Buckland
& Zucchini 2002). However, some populations prove
particularly problematic, in which case indirect surveys
© 2003 British
Ecological Society
*Present address: 24 Maiden Lane Centre, Lower Earley,
Reading RG6 3HD, UK.
Correspondence: S. T. Buckland, Centre for Research into
Ecological and Environmental Modelling, The Observatory,
Buchanan Gardens, St Andrews KY16 9LZ, UK. E-mail
steve@mcs.st-and.ac.uk
of their signs may be easier. Examples include mammals
that are difficult to detect in closed habitats but leave
dung piles that are more amenable for survey (e.g. deer,
elephants and foxes), and mammals that are too scarce
to survey by direct means but leave relatively numerous
signs that can be surveyed (e.g. cat scats, otter spraints
and ape nests).
Surveys of signs measure usage of the survey area over
a period of time, corresponding roughly to the mean
time to decay of the signs. In contrast, direct methods
usually estimate animal density at the time of the survey,
which may be more prone to sample error (Jachmann
1991). Dung methods yield estimates of abundance
that are comparable with estimates using direct methods
1103
Estimating sign
decay rates
© 2003 British
Ecological Society,
Journal of Applied
Ecology, 40,
1102–1111
for a range of species (Barnes 2001), and they have been
found to yield more precise estimates of elephant abundance than aerial sample surveys (Barnes 2002).
Surveys of dung are typically conducted using quadrat
sampling (Bailey & Putman 1981; Putman 1984), strip
transect sampling (Plumptre & Harris 1995) or line
transect sampling (Barnes et al. 1995; Marques et al.
2001). Surveys of ape nests typically use line transect
methods (Plumptre 2000).
To convert estimates of dung or nest density to estimates of animal density, two rates must be estimated:
the production (defecation or nest construction) rate
and the decay or disappearance rate of the dung or
nests. If it is possible to clear the survey plots of signs
before the dung or nest surveys, allowing sufficient time
for new signs to accumulate but not sufficient time for
them to decay, then there is no need to estimate decay
rates (see below).
Production rate can be estimated by following animals or animal groups, by monitoring captive animals,
or by placing a known number of animals in an enclosure, previously cleared of signs, and estimating the
number of signs produced over a fixed time period.
There are many practical problems associated with
estimating production rate. For example rates may vary
seasonally, so care is needed to estimate rates relevant
to the surveys of dung or nests; rates may vary between
animals, so a representative sample of animals should
be monitored; captive animals may exhibit different
rates from wild animals; it may be difficult or impossible
to follow animal groups. Appropriate methods depend
on the study population of interest. In contrast, a generic
approach is possible for estimating decay rates, and in
this paper we propose such an approach that provides
robust estimation of decay rate.
Typically, dung and nest decay rates have been estimated
by assuming an exponential rate of decay (McClanahan
1986; Barnes & Jensen 1987), by estimating an ‘instantaneous mortality rate’ of dung (Barnes & Barnes
1992) or by putting down or locating fresh dung and
monitoring it until it has decayed (Plumptre & Harris
1995; Marques et al. 2001). In this paper, we develop
the suggestions of Marques et al. (2001) and Buckland
et al. (2001). They note that, to convert sign density to
animal density, it is necessary to estimate the mean time
to decay of signs that are present at the time of the survey. A simple way to achieve this is to locate and mark
fresh signs on several dates in the lead up to the survey,
chosen so that the proportion of signs surviving from
the earliest date to the survey is expected to be small,
and to return to marked signs just once, at the time of
the survey. Data are then binary, recording whether or
not the signs survived to the survey. The method of
Hiby & Lovell (1991) is also based on this idea. We term
the resulting estimates ‘retrospective’ estimates of the
mean time to decay, because a time point is identified
and the mean time to decay of signs already present is
estimated. In contrast, most workers identify or lay
down fresh signs at a selected time point, then return
regularly to record when the signs disappear. This gives
a ‘prospective’ estimate of the mean time to decay; if
decay rates vary seasonally, prospective estimates are
biased estimates of the required mean time to decay
because they do not estimate the mean time to decay of
the signs that are present at the time of the survey to
estimate sign density. Further, the prospective method
requires repeat visits to marked signs. However, this
disadvantage is not as great as might be thought, as the
design of a retrospective survey often requires repeat
visits to identify fresh signs or to allow estimation of
decay rate at different times of the year.
We illustrate the methods of this paper using data on
red deer Cervus elaphus L. and roe deer Capreolus capreolus L. populations at Abernethy Forest in Scotland, for
which the management aim is to maintain deer populations at levels that allow natural regeneration of
native Scots pinewood Pinus sylvestris L.
Methods
Density of animals Da is estimated as:
Da =
Ds
π×t
eqn 1
where Ds is the estimated density of animal signs in the
study area, t is the estimated mean time to decay of the
animal signs present when the survey to estimate sign
density is conducted, and π is the estimated rate of production of signs per animal during the period preceding the survey. To quantify precision of the estimate of
animal density, it is important to estimate the precision
of each of the three components of equation 1 (Plumptre
2000). Thus:
[cv(Da )] 2 ≈ [cv( Ds )] 2 + [cv( t )] 2 + [cv( π ) ] 2
eqn 2
where cv(t) is the coefficient of variation of t, defined as
its standard error divided by itself, and similarly for
other terms. Typically, the contribution of variability in
estimated sign density dominates this expression. We
assume that estimates Ds and π are available, together
with their standard errors, and consider here the problem of estimating mean time to decay.
The most extensive work on decay rates has been
conducted on dung piles of forest elephants in Africa.
Short (1983), Merz (1986), McClanahan (1986) and
Barnes & Jensen (1987) all estimate prospective rates,
assuming that the system is in a steady state throughout
the period of the decay experiment. The steady-state
assumption states that the number of dung piles being
deposited each day equals the number disappearing
each day, i.e. the number of dung piles per unit area
remains constant from day to day. This assumption
means that we can estimate mean time to decay of dung
1104
S. E. Laing et al.
© 2003 British
Ecological Society,
Journal of Applied
Ecology, 40,
1102–1111
piles present at the survey by the reciprocal of the daily
rate of decay. Further, if we assume an exponential rate
of decay, then rate of decay is independent of age and
we can monitor any dung pile, not just fresh piles, to
estimate the rate of decay.
Grimshaw & Foley (1990) and Reuling (1991) found
that decay rates were not well modelled by the exponential distribution, with typically slower rates of decay
when the dung piles were fresh. Barnes & Barnes (1992)
therefore reanalysed their data using six different
methods for calculating the mean decay rate from
the data. They confirmed that methods that assume a
constant exponential rate of decay are substantially
biased.
A further major problem with the above methods,
however, is that if the steady-state assumption does not
hold, bias can again be substantial. Seasonal changes
in defecation rates, dung decay rates and elephant
distribution all violate the steady-state assumption
(McClanahan 1986). Even when methods allow decay
rate to vary with the age of the dung pile, the above
methods yield a prospective rate, whereas equation 1
requires that we have a retrospective rate. The two rates
may differ substantially when the steady-state assumption fails. Hiby & Lovell (1991) derived a method that
correctly estimates the retrospective rate, and also
provide software () to allow managers to
estimate elephant abundance.
Hiby & Lovell (1991) noted that the dung piles visible on a survey represent the remains of the dung piles
deposited by elephants in the area over the period preceding the survey, whether or not steady state has been
reached. The decay experiment is therefore directed
towards estimating the proportion of the dung piles
deposited at different times during this period that
remain visible on the date of the survey and, similarly,
the production rate experiment estimates the defecation rate over this period. If elephant density is varying,
they show that equation 1 provides a weighted average
of density in the period preceding the dung survey, with
greater weight being given to the dates immediately
preceding the survey (and to dates on which defecation
rates are high, if these vary).
If decay rates are independent of date, and of other
factors such as sign size, then the prospective and
retrospective rates are the same. However, if seasonal
variation occurs, perhaps because of rainfall patterns
or diet changes, then prospective rates give biased
estimates of animal density. Furthermore, work on deer
pellet groups (B.A. Mayle & A.J. Pearce, unpublished
data, quoted in Marques et al. 2001) indicates that the
average time for a pellet group to decay is a function of
the initial number of pellets in a group, so that groups
present at the time of the survey are a size-biased selection
of groups deposited. The retrospective method of estimating mean time to decay is unaffected by this sizebiased selection, but the prospective method generates bias,
because too few large, long-lived groups and too many
small, short-lived groups are represented in the sample.
Given the above considerations, it is advisable to conduct decay experiments prior to every survey, unless
sufficient data have been collected to allow a reliable
model to be developed that can be shown to predict
mean decay times successfully, given data such as rainfall and habitat for the study area (Barnes et al. 1997;
Barnes & Dunn 2002). In addition, if surveys to estimate animal density from sign density are carried out
over an extended time period, it may be necessary to
make repeated visits to marked animal signs so that the
mean time to decay can be estimated for different times
of the year. Careful survey design may avoid this need.
For example, different strata within the study area may
be surveyed at different times, and the marked signs
within each stratum can be revisited at the time of the
survey in that stratum. The dates of the revisits then vary
by stratum, but each marked sign is only revisited once.
Decay refers to the disappearance of the animal signs
irrespective of the mechanism by which the process
occurred. For example, deer pellet groups that have been
covered by leaves, that have been spread out over a large
area as a result of trampling by the deer, or that have
undergone organic decay are all considered to have
‘decayed’ (Marques et al. 2001). It is important that
the criterion for determining whether a sign has decayed
in the decay rate experiment is the same as that used
in the survey to estimate sign density. For deer pellet
groups, this is typically taken to be the point at which the
number of identifiable pellets falls below some threshold. For dung surveys more generally, it is usually possible to identify stages of decay or disappearance. In
that case, the criterion can be chosen to correspond to
a change from one stage to the next that is relatively
unambiguous.
Following Buckland et al. (2001), we recommend
that a time period is estimated over which 90% or more
of the signs are expected to have met the criterion that
defines ‘decay’. This might be estimated from past data
from the study area or from similar studies elsewhere. If
a time period that is too long is chosen, field costs will
be higher than necessary but estimation of mean time
to decay is not compromised. If the time period is too
short, bias can be anticipated in estimation of mean
time to decay, and hence in estimated animal abundance. Searches for fresh signs should commence this
length of time ahead of the sign survey. A criterion will
be needed for identifying fresh signs. If, for example, a
criterion was shown by experiment to identify a sign as
‘fresh’ if it was up to 4 days old, a fresh sign should be
considered to be 2 days old (the average age of signs
identified as fresh) for purposes of analysis. Each fresh
sign located should be marked to ensure that it can be
accurately relocated.
There should be at least five or six visits to the study
area to search for fresh signs, roughly evenly spaced in
time between the first visit and the subsequent survey
from which sign density is estimated. Thus if it was
1105
Estimating sign
decay rates
judged that at least 90% of signs decay within 6 months,
six monthly searches might be conducted, the last being
a month before the sign survey. At each visit, every
effort should be made to ensure that a representative
sample of fresh signs is located. Ideally, this will involve
a designed survey, for example comprising several
strip transects, randomly or systematically placed
within the study area, that ensures that habitat is
sampled in proportion to its occurrence. The length of
each transect would then be surveyed, and any fresh
sign marked. This ensures that more signs are monitored in areas heavily used by the study species, in proportion to the density of signs, as required for unbiased
estimation.
Because variability in estimated decay rate will
typically be smaller than variability in estimated density
of signs, large sample sizes of fresh signs are not needed.
Buckland et al. (2001) suggest a minimum of 50 in the
monitoring experiment, and Hiby & Lovell (1991)
show by approximate calculation that the contribution
of decay rate estimation to overall variability in the animal abundance estimate is small if the number of monitored signs is of the order of 100.
environmental improvement. Although each respondent is offered a single bid level, different respondents
are offered different bids, according to some design.
This leads to a binary response of 1 if the bid is accepted
or 0 if the bid is refused. If we regard the animal sign as
the ‘respondent’, and the length of time between identification of a fresh sign and the subsequent revisit as
the ‘bid level’, then the same methods can be used for
modelling decay rate. A sign still present at the revisit
corresponds to accepting the bid.
For animal sign i, i = 1, … , n, we define the random
variable Yi to be 1 if the sign is judged not to have
decayed at the revisit, or 0 otherwise, and we denote the
time between production of sign i and the revisit by xi,
which is therefore the age of the sign at the time of the
revisit, if it has survived. Then:
E(Yi | xi ) = Pr(Yi = 1 | xi ) = p (xi )
where p (xi ) is the probability that sign i survives until
the revisit, assumed for the moment to depend on xi
alone. We assume:
E (Yi | xi ) = p ( xi ) =
Hiby & Lovell (1991) do not specify how they analyse
data from an experiment of this type. Perhaps the most
obvious option is logistic regression, and we outline
this approach, together with possible transformations
to improve model fit, below.
The following methods are based on Buckland et al.
(1999), who developed methods for estimating mean
willingness to pay for some environmental goal. In that
case, a survey is conducted such that a number of respondents must state whether they would be willing to
pay a fixed sum of money to achieve a specified level of
© 2003 British
Ecological Society,
Journal of Applied
Ecology, 40,
1102–1111
1
1 + exp{− ( β0 + β1xi )}
eqn 3
where β0 and β1 are coefficients to be estimated.
Let the random variable X represent the lifetime of
an animal sign (i.e. the length of time until the sign is
judged to have decayed). The quantity of interest is
then the mean time to decay, which we denote µX.
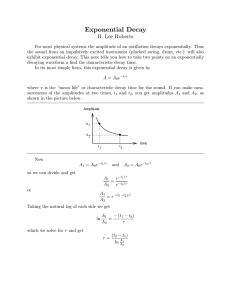
Figure 1 shows a diagrammatic representation of the
decay experiment.
Estimation of mean time to decay and the corresponding variance are covered in the Appendix. Note
that the logistic function of equation 3 is defined over
the full range of x, from –∞ to ∞, whereas in the current
Fig. 1. Diagrammatic representation of the decay experiment. Pi represents the time at which sign i was produced, and Gi the
point in time at which sign i decayed. Sign 1 was therefore still present at the time of the revisit, but sign 2 had decayed.
1106
S. E. Laing et al.
context x is constrained to be non-negative. One solution is to left-truncate the distribution at x = 0 (see the
Appendix). Another solution is to replace x in our
model by loge(x), so that the range on x of (0, ∞) transforms to a range on loge(x) of (– ∞, ∞), giving a logistic
regression over the full range of the real line. The model
is then:
p (x ) =
1
1 + exp {− ( β0 + β1 log e (x ))}
eqn 4
Again, results for mean time to decay are deferred to
the Appendix.
Although the logarithmic transformation ensures
that negative ages are impossible, it can also create
problems for some data sets when fitting the upper tail
of the logistic curve. In particular the upper tail may
be considerably lengthened by the logarithmic transformation, which can result in estimates of mean time to
decay that are biased high. A solution to this problem is
to identify a transformation that does not alter behaviour of the upper tail. One such transformation is the
reciprocal transformation, where x is replaced by w = x
– β2/x for some β2. The general logistic equation given
by equation 3 is a limiting case of this transformation
as β2 → 0. The value of β2 might be fixed arbitrarily, but
it is better considered an unknown parameter to be
estimated. The regression is carried out as before, but with
the additional term β2/x. Equation 3 now becomes:
p (x ) =
1
1 + exp {− ( β0 + β1 x + β 2 /x )}
eqn 5
with β1 < 0 and β2 > 0. See the Appendix for estimating
mean time to decay under this model.
Suppose covariates such as habitat type and rainfall
are recorded, in addition to age of the sign. The logistic
regression equation may be expressed as:
E ( yi ) =
1
1 + exp − β0 − ∑ β j xij
j
eqn 6
where xij is the value of covariate j for animal sign i, j ≥
1, and βj are coefficients to be estimated, j ≥ 0.
If a number of potential covariates are recorded,
stepwise methods may be used to reduce the number
of covariates in the model. Age xi1 (and / or its transformation where relevant) should always be retained in
the model.
The corresponding fitted model may now be expressed as:
¥i =
© 2003 British
Ecological Society,
Journal of Applied
Ecology, 40,
1102–1111
1
1 + exp − b0 − ∑ b j xij
j
eqn 7
Each sign now has a unique estimated decay curve, so
that estimation of mean time to decay is less straight-
forward. The solution proposed by Buckland et al. (1999)
is to calculate the prediction ¥i for each animal sign i,
using equation 7. These predictions are sign-specific
estimates of the probability that the sign has decayed by
the time of the revisit. They can be plotted against x1i,
the time between production of sign i and the revisit. A
logistic curve can be fitted to the plot, assigning a weight
1/{¥i (1 − ¥i )} to ¥i. [In the willingness-to-pay application of Buckland et al. (1999), the distribution of bid
level was discrete whereas that of x1i is continuous, except
for the effect of rounding to, say, whole days, so we have
modified the proposed method slightly.]
This logistic curve differs from the logistic regressions described earlier, because the logistic regressions
assumed that the data were from a binomial distribution
and the curves were fitted using iterative reweighted
least squares. Here, the logistic curves are fitted using
non-linear weighted least squares.
If we denote the logistic curve as:
p ( x1 ) =
1
{
1 + exp − ( β0 + β1x1 )}
eqn 8
then this has exactly the same form as equation 3. The
fitted logistic curve yields estimates b1 and b2 of β1 and
β2, allowing us to estimate the mean time to decay, 8X,
and its corresponding variance as previously. The
approach is readily extended to allow the logarithmic
transformation of x, or the addition of a term in 1/x.
Example: red and roe deer surveys in Scotland
Fifteen plots were established and cleared of dung
during January 2000 in Abernethy Forest in Scotland.
Each plot was surveyed on average on 10 occasions
between January 2000 and May 2001, with intervals
between visits of between 6 and 8 weeks. Any new pellet
groups were marked and the species of deer recorded.
The estimated date of deposit of each pellet group was
taken as 24 days before the date that it was marked (i.e.
roughly half the time elapsed between the date of marking and the date of the previous visit). For each pellet
group, its date of disappearance was recorded. A pellet
group was considered to have decayed if less than six
identifiable pellets remained. The estimated date of decay
was taken as 24 days before the date of the first visit
for which the pellet group was judged to have decayed.
As a number of pellet groups had not decayed during
the period of monitoring, the date when the pellet
group was last checked was recorded for these pellet
groups.
Line transect surveys of dung were conducted in
May 2001. Thus only a single observation was required
for each marked sign, whether or not it was still present
in May 2001. These observations were readily obtained
from the observed dates of decay, which were recorded
for research purposes.
1107
Estimating sign
decay rates
Table 1. Results from fitting the three logistic models to the red deer pellet group data
No transformation
Log transformation
Reciprocal transformation
Model
Residual deviance
Null
+ age
+ habitat
Null
+ log e (age)
+ habitat
Null
+ age
+ 1 / age
+ habitat
68·744
18·867
15·862
68·744
18·165
15·518
68·744
18·867
17·931
15·181
Change in deviance
49·877
3·184
50·579
2·647
49·877
0·936
2·749
d.f.
Change in d.f.
P
53
52
50
53
52
50
53
52
51
49
1
2
< 0·0001*
0·203
1
2
< 0·0001*
0·266
1
1
2
< 0·0001*
0·333
0·253
d.f.
Change in d.f.
P
1
2
< 0·0001*
0·228
1
2
< 0·0001*
0·331
1
1
2
< 0·0001*
0·866
0·230
*Significant at the 5% significance level.
Table 2. Results from fitting the three logistic models to the roe deer pellet group data
No transformation
Log transformation
Reciprocal transformation
Model
Residual deviance
Null
+ age
+ habitat
Null
+ log e (age)
+ habitat
Null
+ age
+ 1 / age
+ habitat
98·922
51·053
48·092
98·922
57·259
55·046
98·922
51·053
51·024
48·082
Change in deviance
47·870
2·960
41·663
2·213
47·870
0·029
2·942
71
70
68
71
70
68
71
70
69
67
*Significant at the 5% significance level.
© 2003 British
Ecological Society,
Journal of Applied
Ecology, 40,
1102 –1111
The survey date chosen as the fixed reference date for
the logistic regression analysis of the red and roe deer
data sets was 15 May 2001. The status of the pellet group
at this reference date was determined: if the estimated
date of decay was after the fixed reference date (or if the
pellet group was still present at the final visit), then the
status of the pellet group was recorded as 1; otherwise,
it was recorded as 0.
For each pellet group, habitat was recorded as a
factor at three levels. There were in total 54 and 72
observations (pellet groups) for red deer and roe deer,
respectively, after deleting those for which habitat had
not been recorded.
Three logistic models, each incorporating the habitat
covariate, were fitted to the data. Model 1 was the lefttruncated logistic model with no transformation of the
age variable; model 2 was the logistic model with the log
transformation of the age variable; model 3 was the
logistic model with the reciprocal transformation of the
age variable. The results from fitting each model appear
in Table 1 (red deer) and Table 2 (roe deer). In each case,
a null model was fitted first, then a model incorporating sign age, and finally a model incorporating habitat
as well. For both species, no evidence of an effect of
habitat was found, and the left-truncated model without
transformation of x proved adequate. Use of loge(x)
reduced the error deviance appreciably less for roe deer
and slightly more for red deer than use of untransformed
x, and the term in 1/x was not significant for either species. Therefore, our favoured option for roe deer was
the left-truncated model without the habitat covariate,
and for red deer either this model or the model with
loge(x) and no habitat covariate appeared satisfactory.
We thus dropped the covariate and used the straightforward logistic regression methods for estimating
mean time to decay. For comparative purposes, we
also show results for the models with a logarithmic
and a reciprocal transformation of x, also without the
covariate.
Figures 2 and 3 show the fitted logistic regression
curves under all three models, excluding the habitat
covariate, for red deer and roe deer, respectively. The
models yielded similar fits to these data. The estimated
mean time to decay, 8X, its approximate standard error,
SE (8X ) and 95% log-normal confidence interval for all
three models are shown in Table 3. Higher variability
was evident under model 2, reflecting the greater uncertainty associated with a wider upper tail for the model
when age was log-transformed, and high variability
was shown by model 3 for red deer only, but generally
the different models yielded very similar estimates of
mean time to decay for each species.
1108
S. E. Laing et al.
Table 3. Estimated mean time to decay, 8X, its standard error,
SE ( 8X ), and 95% log-normal confidence interval. In model 1,
x is left-truncated and untransformed; in model 2, x is logtransformed; in model 3, a term in 1/x is added to model 1
Species
Model
8X
SE ( 8X)
Log-normal 95%
confidence interval
Red deer
Red deer
Red deer
Roe deer
Roe deer
Roe deer
1
2
3
1
2
3
295
280
275
260
260
252
31
39
42
25
34
26
240, 362
213, 369
204, 371
215, 315
202, 334
206, 308
Discussion
Methods for estimating the density of signs (usually
dung or nests), for example using quadrat sampling,
strip transect sampling or line transect sampling, are
well developed and understood. There is greater difficulty in estimating the two rates that allow sign density
to be converted to animal density: sign production rate
and the decay rate (or equivalently its reciprocal, the
mean time to decay). In this paper, we address the latter
problem. Estimation of the production rate must be
addressed on a case-by-case basis, as methods suitable
for some populations are not suitable for others. For
more difficult populations there would seem to be
considerable scope for developing electronic methods to
monitor a sample of animals remotely.
The methods developed here can be readily implemented using standard statistical software that provides
logistic regression and logistic curve fitting facilities,
together with numerical integration. Cameron (1988)
adopted a strategy for modelling willingness to pay
without including bid level (in our context, age of sign)
as a covariate. Instead, Cameron (1988) developed a
censored logistic regression approach. However, her
more direct approach requires methods that are not
available in standard statistical software.
The sign survey methods assumed in this paper are
often called ‘standing crop’ methods, because the survey
to estimate sign abundance records all detected signs
Fig. 2. The logistic regression curves fitted to the red deer pellet group data. The open circles show the observed data, which are
1 for pellet groups surviving to 15 May 2001 and 0 otherwise.
© 2003 British
Ecological Society,
Journal of Applied
Ecology, 40,
1102–1111
Fig. 3. The logistic regression curves fitted to the roe deer pellet group data. The open circles show the observed data, which are
1 for pellet groups surviving to 15 May 2001 and 0 otherwise.
1109
Estimating sign
decay rates
on the survey plots, irrespective of age (unless they are
still detectable but are deemed to have decayed). In contrast, ‘clearance plot’ methods avoid the need to have to
estimate decay rates. Survey plots are cleared of any
signs, and are then revisited before any new signs have
had time to decay. The amount of sign deposited per day
within the survey region is estimated from the resulting
data; this estimate is divided by an estimate of the deposition rate per animal per day, to yield estimated animal abundance. The clearance plot method is generally
regarded as efficient only when animal density is high
(Buckland 1992). It has the substantial advantage over
the standing crop method of not requiring an estimate
of decay rate. Thus abundance can be estimated relatively quickly, without the need to monitor signs over a
lengthy time period. Its disadvantages over the standing crop method are as follows. Decay rates tend to be
highly variable, so that the time period between visits
must be short to ensure that new signs do not decay
before the site is revisited. This means that many more
sampled plots (or larger plots) must be surveyed to
allow estimation of sign abundance with comparable
precision to that achievable with standing crop methods.
[Typically, precision on this estimate dominates precision of the final animal abundance estimate (Plumptre
2000).] Added to this, the sampled plots must be cleared
of all signs at the outset, and accurately relocated during
the survey of signs, whereas the standing crop method
can use distance sampling methods (Buckland et al.
2001) for efficient estimation of dung abundance, without the need to locate all signs on the sampled plots and
without the need for marked plots. Except at high densities, the advantage of not having to search for fresh
signs from which to estimate decay rates, or to monitor
the signs over time, is usually more than offset by these
disadvantages, especially as a larger sample of signs is
needed to estimate the mean number of signs deposited
per day throughout the survey region with comparable precision to that for estimates of mean decay
rate. A further factor in favour of the standing crop
method is that it may often prove possible to develop
a model to predict decay rate so that a decay rate experiment is not needed in every survey site at every time
point.
Investigation into how high the density should be for
the clearance plot method to be more cost-effective than
the standing crop method would be useful. However,
conclusions will vary appreciably between studies
and species.
Acknowledgements
© 2003 British
Ecological Society,
Journal of Applied
Ecology, 40,
1102 –1111
We thank the Royal Society for the Protection of Birds,
who conducted the surveys and funded the development of the survey design. Analyses were conducted for
the dissertation element of the first author’s MSc,
which was funded by BBSRC. We also thank Richard
Barnes for his comments on an earlier draft, and two
referees for their supportive comments.
References
Bailey, R.E. & Putman, R.J. (1981) Estimation of fallow deer
Dama dama populations from faecal accumulation. Journal
of Applied Ecology, 18, 697–702.
Barnes, R.F.W. (2001) How reliable are dung counts for
estimating elephant numbers? African Journal of Ecology,
39, 1–9.
Barnes, R.F.W. (2002) The problem of precision and trend
detection posed by small elephant populations in West
Africa. African Journal of Ecology, 40, 179–185.
Barnes, R.F.W. & Barnes, K.L. (1992) Estimating decay rates
of elephant dung-piles in forest. African Journal of Ecology,
30, 316 –321.
Barnes, R.F.W. & Dunn, A. (2002) Estimating forest elephant
density in Sapo National Park (Liberia) with a rainfall
model. African Journal of Ecology, 40, 159–163.
Barnes, R.F.W. & Jensen, K.L. (1987) How to count elephants
in forests. IUCN African Elephant and Rhino Specialist
Group, 1, 1–6.
Barnes, R.F.W., Asamoah Boateng, B., Majam, J.N. & Agyei
Ohemeng, J. (1997) Rainfall and the population dynamics
of elephant dung-piles in the forests of southern Ghana.
African Journal of Ecology, 35, 39–52.
Barnes, R.F.W., Blom, A., Alers, M.P.T. & Barnes, K.L.
(1995) An estimate of the number of forest elephants in
Gabon. Journal of Tropical Ecology, 11, 27–37.
Borchers, D.L., Buckland, S.T. & Zucchini, W. (2002)
Estimating Animal Abundance: Closed Populations.
Springer Verlag, London, UK.
Buckland, S.T. (1992) Review of Deer Count Methodology. Report
to Scottish Office Agriculture and Fisheries Department, UK.
Buckland, S.T., Anderson, D.R., Burnham, K.P., Laake, J.L.,
Borchers, D.L. & Thomas, L. (2001) Introduction to Distance
Sampling: Estimating Abundance of Biological Populations.
Oxford University Press, Oxford, UK.
Buckland, S.T., Macmillan, D.C., Duff, E.I. & Hanley, N.
(1999) Estimating mean willingness to pay from dichotomous choice contingent valuation studies. Statistician, 48,
109 – 124.
Cameron, T.A. (1988) A new paradigm for valuing nonmarket goods using referendum data: maximum likelihood
estimation by censored logistic regression. Journal of
Environmental Economics and Management, 15, 355–379.
Grimshaw, J.M. & Foley, C.A.H. (1990) Kilimanjaro Elephant
Project 1990: Final Report. Friends of Conservation, Nairobi.
Hiby, L. & Lovell, P. (1991) – a program for estimating elephant density from dung density without assuming
‘steady state’. Censusing Elephants in Forests: Proceedings
of an International Workshop (eds U. Ramakrishnan,
J.A. Santosh & R. Sukumar), pp. 73–79. Asian Elephant
Conservation Centre, Bangalore, India.
Jachmann, H. (1991) Evaluation of four methods for estimating
elephant densities. African Journal of Ecology, 29, 188–195.
McClanahan, T.R. (1986) Quick population survey method
using faecal droppings and a steady state assumption.
African Journal of Ecology, 24, 37–39.
Marques, F.F.C., Buckland, S.T., Goffin, D., Dixon, C.E.,
Borchers, D.L., Mayle, B.A. & Peace, A.J. (2001) Estimating deer abundance from line transect surveys of dung: sika
deer in southern Scotland. Journal of Applied Ecology, 38,
349 – 363.
Merz, G. (1986) Counting elephants (Loxodonta africana
cyclotis) in tropical rain forests with particular reference to
the Tai National Park, Ivory Coast. African Journal of Ecology, 24, 61 – 68.
Plumptre, A.J. (2000) Monitoring mammal populations with
line transect techniques in African forests. Journal of
Applied Ecology, 37, 356 –368.
Plumptre, A.J. & Harris, S. (1995) Estimating the biomass of
large mammalian herbivores in a tropical montane forest –
1110
S. E. Laing et al.
a method of faecal counting that avoids assuming a steadystate system. Journal of Applied Ecology, 32, 111–120.
Putman, R.J. (1984) Facts from faeces. Mammal Review, 14,
79–97.
Reuling, M.A. (1991) Elephant use of the Marang Forest
Reserve in Northern Tanzania. MSc Thesis. University of
Washington, Seattle.
Seber, G.A.F. (1982) The Estimation of Animal Abundance and
Related Parameters, 2nd edn. Griffin, London, UK.
Short, J.C. (1983) Density and seasonal movements of the forest elephant (Loxodonta africana cyclotis Matschie) in Bia
National Park, Ghana. African Journal of Ecology, 21, 175–184.
Received 31 March 2003; final copy received 27 August 2003
Appendix
We need to evaluate µX = E(X ). If animal sign i is still present at the time of the survey, then X must be greater than
xi, i.e. p(xi) = Pr(X > xi) (see Fig. 1). Hence the cumulative distribution function of X, F (x), is given by:
F (x) = 1 − Pr (X > x) = 1 − p (x)
eqn 9
The probability density function f (x) is then obtained by differentiating with respect to x:
f ( x ) = F ′( x ) = −
d
p (x )
dx
eqn 10
The mean time to decay, µX = E(X ), can then be calculated as:
∞
µ X = E (X ) =
x f (x ) d x
eqn 11
–∞
If we left-truncate the distribution at x = 0, we must rescale f(x) so that it again integrates to unity. This solution
to the problem of negative x works well when F(0) for the untruncated distribution is close to 0, and we develop this
approach before considering transformations that offer a more satisfactory mathematical solution.
Applying the result of equation 10 to equation 3, we obtain:
f (x ) =
−β1 exp [ − ( β0 + β1x )]
[1 + exp{− ( β0 + β1x )}] 2
eqn 12
where β1 < 0. However:
∞
f (x ) d x = 1 + exp1 (−β )
eqn 13
0
0
To ensure that f(x) integrates to 1 after left-truncating at 0, equation 12 must be divided by equation 13. The
modified logistic curve is then given by:
f (x ) =
−β1 [1 + exp ( − β0 )] exp [ − ( β0 + β1x )]
[1 + exp{− ( β0 + β1x )}] 2
eqn 14
from which:
∞
µX =
−β x[1[1+ +expexp{(−β−()]β exp+ β[ −x(β)}] + β x)] d x
1
0
0
1
eqn 15
2
0
1
0
Estimates b0 and b1 obtained from the logistic regression of Y on x are substituted for β0 and β1, respectively, in
equation 15. The estimate for µX, 8X, is then obtained by numerical integration of equation 15. The delta method
(Seber 1982) yields the following approximate result:
2
© 2003 British
Ecological Society,
Journal of Applied
Ecology, 40,
1102–1111
∞
∞
uxb [u − 1 − 2 × exp( −b )]
ux{1 + exp( − b 0 )}{xb 1 (u − 1) + u + 1}
1
0
ˆ
ˆ
ˆ
d x
Var( µˆ X ) = Var ( b 0 ) −
d x + Var ( b 1) −
(1 + u )3
(1 + u )3
0
0
2
∞
∞
ˆ ( b 0, b 1) uxb 1 [ u − 1 − 2 × exp( −b 0 )] d x ux{1 + exp( − b 0 )}{xb 1 (u − 1) + u + 1} d x
+ 2Cov
(1 + u ) 3
(1 + u )3
0
0
eqn 16
1111
Estimating sign
decay rates
where u = exp{−(b0 + b1x)}
The integrals are evaluated by numerical integration, and standard logistic regression packages give the variances
and covariance of the coefficients.
If we replace x in our model by loge(x) (equation 4), then we obtain:
∞
µX =
exp{− ( β + β log ( x ) )}
dx
[1−β+ exp{
− ( β + β log ( x ) )}]
1
0
1
e
eqn 17
2
0
1
e
0
The estimates b0 and b1 obtained from the logistic regression of Y on loge(x) are substituted for β0 and β1, respectively, and 8X is again obtained by numerical integration. The estimated variance is now:
2
∞
∞
ub (u − 1)
u{( u − 1)b log ( x ) + u + 1}
1
1
e
ˆ
ˆ
ˆ
Var ( µˆ X ) = Var ( b 0 ) −
d x + Var ( b 1 ) −
d x
(1 + u )3
(1 + u )3
0
0
2
∞
eqn 18
∞
ˆ ( b 0, b 1 ) ub1 ( u − 1) d x
+ 2Cov
(1 + u )3
u{(u − 1)b(1log+ u()x ) + u + 1} d x
1
e
3
0
0
where u = exp{−(b0 + b1 log e(x))}
If instead we add a term β2/x to our model, then:
∞
µX =
− ( β + β x + β /x )}
dx
−x(β[1 −+ βexp{/x −)(exp{
β + β x + β /x )}]
2
1
2
0
0
1
1
2
2
eqn 19
2
0
The estimates b0, b1 and b2 obtained from a logistic regression of Y on the two variables x and 1/x, are substituted
for β0, β1 and β2, respectively. The estimate 8X is then obtained by numerical integration of equation 19. The variance of this estimate is estimated as:
2
∞
∞
xu ( b 1 − b 2 /x 2 )( u − 1)
ux [ x ( u − 1)( b 1 − b 2 /x 2 ) + (1 + u )]
Var( µˆ X ) = Var ( b 0 ) −
d x + Var ( b 1 ) −
d x
(1 + u )3
(1 + u )3
0
0
2
∞
u [ x ( b 1 − b 2 /x 2 )(u − 1) − (1 + u )]
+ Var ( b 2 ) −
d x
x (1 + u )3
0
2
∞
∞
xu ( b 1 − b 2 /x 2 )( u − 1)
+ 2Cov ( b 0, b 1)
dx
(1 + u )3
0
∞
ux[x(u − 1)( b(1 −+ bu )/x ) + (1 + u )] d x
2
1
2
3
0
∞
ux [ x ( u − 1)( b 1 − b 2 /x 2 ) + (1 + u )]
u [x ( b 1 − b 2 /x 2 )(u − 1) − (1 + u )]
+ 2Cov ( b 1, b 2 )
dx
dx
3
x (1 + u )3
(1 + u )
0
∞
+ 2Cov ( b 0, b 2 )
∞
0
where u = exp{−(b0 + b1x + b2 /x)}
© 2003 British
Ecological Society,
Journal of Applied
Ecology, 40,
1102 –1111
0
xu ( b 1 − b 2 /x )( u − 1)
u [ x ( b 1 − b 2 /x 2 )( u − 1) − (1 + u )]
dx
d
x
x (1 + u )3
(1 + u )3
2
0
eqn 20