Infrared sensors
advertisement
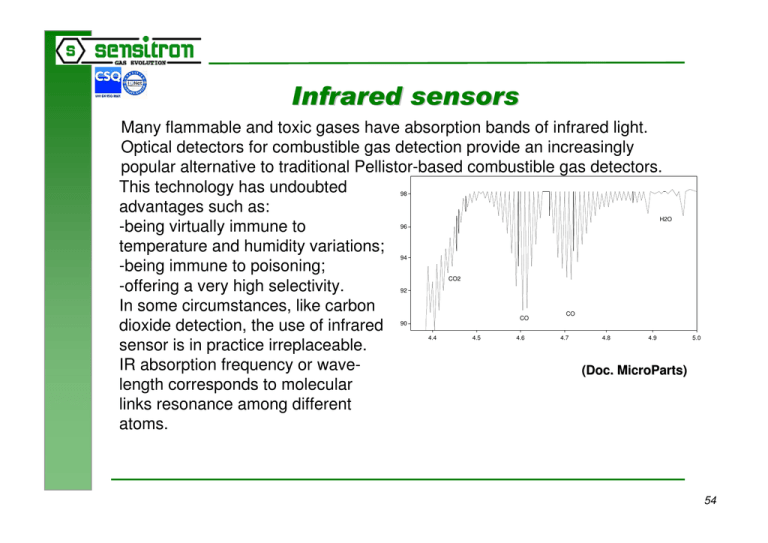
$- ''' Many flammable and toxic gases have absorption bands of infrared light. Optical detectors for combustible gas detection provide an increasingly popular alternative to traditional Pellistor-based combustible gas detectors. This technology has undoubted advantages such as: -being virtually immune to temperature and humidity variations; -being immune to poisoning; -offering a very high selectivity. In some circumstances, like carbon dioxide detection, the use of infrared sensor is in practice irreplaceable. IR absorption frequency or wave(Doc. MicroParts) length corresponds to molecular links resonance among different atoms. 98 H2O 96 94 CO2 92 CO 90 4.4 4.5 4.6 CO 4.7 4.8 4.9 5.0 54 $- ''' ),) In a specific wavelength, optical sensors take the attenuation difference between a reference signal and the signal made by the light passing in the substance to survey. A source of light gives out a ray of light that gets to an optical receiver, through a path fixed in advance and after being filtered. The source of light may be a tungsten bulb, a diode LED or a IR source. According to the type of substance to survey, the optical path will have a different length as far as to use a multireflection system made with adequate mirrors. The sensor may be open or closed in a cuvette, with inlets and outlets for the gas or the mixture to survey. 55 $- ''' ),) The source of light is usually pulsating as sensors require a modulated signal. The reference sensor is usually positioned in a IR band not influenced by the presence of the gas. The sensor may be photovoltaic, photoconductive or pyroelectric. Some optical element may be inserted at the end of the optical path to protect the sensitive part from corrosion. (Doc. Draeger) 56 $- ''' )),' Infrared sensors may be used to survey the most of combustible gas, except hydrogen, in every concentration range up to 100% V/V. Oxygen has no influence on the signal. By selecting adequate wavelength and optical path length, we can obtain apparatus for: • • • • hydrocarbons total measure selective measure of a single component in a mixture ppm measure of small parts measure up to 100% V/V Sensors ranges and characteristics must be fixed on the basis of the application. Compensation of environmental conditions, self-diagnosis and self-calibration qualify the sensor and reduce the maintenance. 57 $- ''' $, Pressure variations have no effects on the zero of the signal but the sensitivity is directly proportional to the pressure. Knowing this sensitivity, it is necessary to avoid pressure alterations at gas entrance. Fast temperature changes may also causes unbalances but, through a proper design, the effect may be minimised. Other gases interfering on the same waveband may cause a sensitivity worsening. Absorptionbands of some gases. Please use all this values for discussion only Gas Absorption in µm and cm-1 (Maxlmum or Range) HC (Hydrocarbons) 3.4 2940 * 6–8 1666 – 1250 CO2 (Carbon dloxide) 4.22 2370 4.26 2347 15 670 N2O(Nitrous oxide) 4.45 2247 4.50 2222 * 7.7 - 7.9 1299 – 1266 CO (Carbon monoxide) 4.6 2174 4.7 2128 H2O (Water) 2.5 - 2.9 4000 – 3450 5–8 2000 – 1250 NH3 (Ammonia) 3.0 3333 * 6.15 1626 9 – 12 1111 - 633 SO2 (Sulfur dioxide) 4.0 2500 * 7.25 1380 * 7.40 1350 8.6 1163 8.8 1136 18 – 20 555 – 500 NOX (Nitric oxide) * 5.3 1887 * 5.4 1852 NO2 (Nitrogen dioxlde) and others, all are not stable under common conditions and can not be measured using simply equipments! ! Estimated Intensity strong middle strong strong middle middle middle weak middle middle middle strong weak middle strong very weak strong strong middle middle weak weak weak (*) Measurement disturbed by water vapour (!) (Doc. MicroParts) 58











